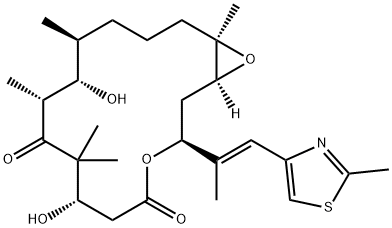
Epothilone B synthesis
- Product Name:Epothilone B
- CAS Number:152044-54-7
- Molecular formula:C27H41NO6S
- Molecular Weight:507.68
![4,17-Dioxabicyclo[14.1.0]heptadec-13-ene-5,9-dione, 7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(1E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-, (1S,3S,7S,10R,11S,12S,13E,16R)-](/CAS/20210305/GIF/595558-95-5.gif)
595558-95-5
0 suppliers
inquiry

152044-54-7
277 suppliers
$50.00/2mg
Yield:152044-54-7 71%
Reaction Conditions:
with TrisNHNH2;triethylamine in 1,2-dichloro-ethane at 50; for 6 h;
Steps:
Compound 51:
To a solution of 49 (0.7 mg, 1. [38] [UMOL)] and TrisNHNH2 (20.6 mg, 69 [UMOL)] in [C1CH2CH2CI] (0.4 [ML)] at [50 °C] was added Et3N (9. 6 μL, 69 mol). The reaction was monitored by HPTLC (hexane/EtOAc = 1/2). After stirring for 6 h, the mixture was cooled to rt, diluted with EtOAc and filtered through a pad of silica gel, which was rinsed with EtOAc. After concentrating, the residue was purified by preparative TLC (hexane/EtOAc= 1/2) gave 51 (0.5 mg, 0.985 μmol, 71%) as a white solid. The spectral data of 51 was identical to those reported of EpoB.
References:
WO2004/18478,2004,A2 Location in patent:Page 83
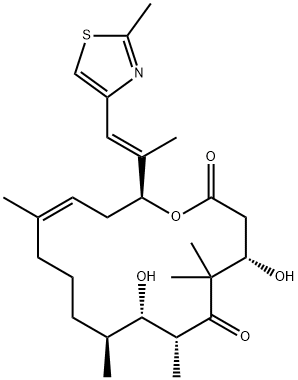
189453-10-9
147 suppliers
$55.00/1mg

152044-54-7
277 suppliers
$50.00/2mg
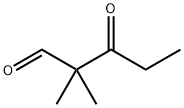
106921-60-2
60 suppliers
inquiry

152044-54-7
277 suppliers
$50.00/2mg
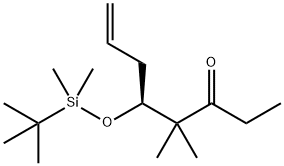
187283-44-9
2 suppliers
inquiry

152044-54-7
277 suppliers
$50.00/2mg
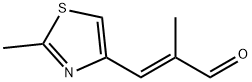
184246-38-6
9 suppliers
inquiry

152044-54-7
277 suppliers
$50.00/2mg