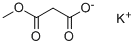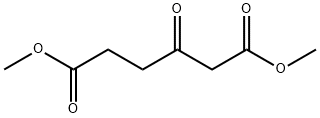
DIMETHYL 3-OXOADIPATE synthesis
- Product Name:DIMETHYL 3-OXOADIPATE
- CAS Number:5457-44-3
- Molecular formula:C8H12O5
- Molecular Weight:188.18
Yield:5457-44-3 70%
Reaction Conditions:
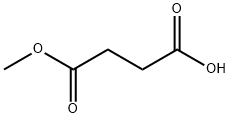
Stage #1: methyl hydrogen succinatewith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 1 h;Inert atmosphere;
Stage #2: monomethyl monopotassium malonatewith magnesium chloride in tetrahydrofuran at 20 - 40; for 13 h;Inert atmosphere;
Steps:
1; 2; 3 Reference Example 1
Preparation of α-Hydromuconic Acid (I-1)
The α-hydromuconic acid used in the present invention was prepared by chemical synthesis.
First, 1.5 L of super-dehydrated tetrahydrofuran (manufactured by Wako Pure Chemical Industries, Ltd.) was added to 13.2 g (0.1 mol) of succinic acid monomethyl ester (manufactured by Wako Pure Chemical Industries, Ltd.), and 16.2 g (0.1 mol) of carbonyldiimidazole (manufactured by Wako Pure Chemical Industries, Ltd.) was added thereto with stirring, followed by stirring the resulting mixture under nitrogen atmosphere for 1 hour at room temperature.
To this suspension, 15.6 g (0.1 mol) of malonic acid monomethyl ester potassium salt and 9.5 g (0.1 mol) of magnesium chloride were added.
The resulting mixture was stirred under nitrogen atmosphere for 1 hour at room temperature, and then stirred at 40° C. for 12 hours.
After the reaction, 0.05 L of 1 mol/L hydrochloric acid was added to the mixture, and extraction with ethyl acetate was carried out.
By separation purification by silica gel column chromatography (hexane:ethyl acetate=1:5), 13.1 g of pure 3-oxohexanedicarboxylic acid dimethyl ester was obtained. Yield: 70%.
References:
US2017/320819,2017,A1 Location in patent:Paragraph 0048; 0049; 0052; 0053; 0056; 0057
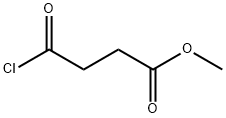
1490-25-1
189 suppliers
$25.00/5g

5457-44-3
40 suppliers
inquiry
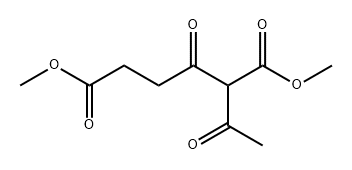
412018-94-1
0 suppliers
inquiry

5457-44-3
40 suppliers
inquiry