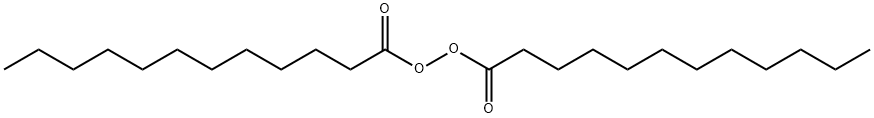
Lauroyl peroxide synthesis
- Product Name:Lauroyl peroxide
- CAS Number:105-74-8
- Molecular formula:C24H46O4
- Molecular Weight:398.62
2C11H23COCl + H2O2+ 2NaOH → (C11H23CO2)2 + 2HCl
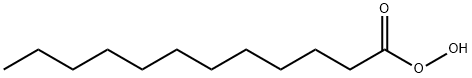
112-16-3
320 suppliers
$13.00/50g

105-74-8
175 suppliers
$35.00/25g
Yield:105-74-8 99.1%
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in 2-Methylpentane;lithium hydroxide monohydrate at 16 - 18; for 0.666667 h;
Steps:
1 Production of Powdered Lauroyl Peroxide
1250 g water, 42 g (0.86 mol) hydrogen peroxide70%, 252 g (1.58 mol) caustic soda lye 25% and 60 g isohexane are provided and cooled to 16° C. 240 g (1.10 mol) lauric acid chloride are added dropwise, over 20 minutes while cooling, at temperatures of from 16 to 18° C. Following the addition, the reaction mixture is stirred for a further 20 minutes at the same temperature. Said mixture is filtered using a Nutsche filter and the solid matter obtained is repeatedly washed using a total of 4 litres of watet 291 g of product damp from water is obtained having a content of 75%, which corresponds to a yield of 98%. After drying in air or in a vacuum, the lauroyl peroxide content was 99.1%. The chlorine content in the product is 60 ppm, the lauric per-acid content is 0.01%.
References:
US2018/44289,2018,A1 Location in patent:Paragraph 0049
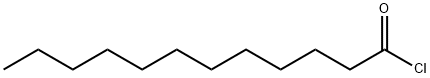
143-07-7
764 suppliers
$5.00/25g

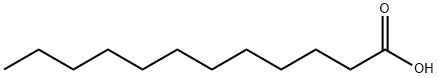
105-74-8
175 suppliers
$35.00/25g
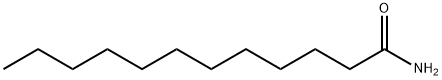
1120-16-7
108 suppliers
$22.00/5g

105-74-8
175 suppliers
$35.00/25g