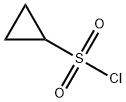
Cyclopropanesulfonyl chloride synthesis
- Product Name:Cyclopropanesulfonyl chloride
- CAS Number:139631-62-2
- Molecular formula:C3H5ClO2S
- Molecular Weight:140.59
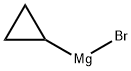
23719-80-4
176 suppliers
$85.20/25ml

139631-62-2
252 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
Stage #1:cyclopropylmagnesium bromide with sulfur dioxide in tetrahydrofuran at -10 - 20; for 0.666667 h;
Stage #2: with N-chloro-succinimide in tetrahydrofuran at -5 - 20;
Steps:
11a.2A
To a solution of cyclopropylmagnesium bromide (0 5 M 20 mL, 10 0 mmol) in anhydrous THF (10 mL) at about -100C is added a solution of SO; in THF (~ 16 wt%, 4 8 mL, 12 mmo.) slowiy over 10 mm at -10 to -5°C Tne reaction mixture is warmed to ambient temperature over 0 5h, and then NCS (2 O g 15 mmo.) is added at about -5 to 0°C The reaction mixture is warmed up to ambient temperature and diluted with 50 mL oi methyl terl-buty. ether To tne reaction mixture ι≤ added 50 mL water and the mixture is stirred for 5 mm The organic layer is washed with 50 mL of bnne The organic layer is concentrated and the resultant cyclopropylsuifony. chloride is dissolved in CH2Ci2 (total volume was about 50 mL) and ammonia gas is bubbled in at about O'C for about 5 mm and the mixture is slowly warmed up to ambient temperature and stored at that temperature for 2 h The mixture is filtered through Cehte to remove the solid (NH4C.), and the filtrate is concentrated to obtain a crude cyclopropyisulfonamide solid (~ 1 2g) Re-cryslailization of the crude product from EtOAc/hexane produces cyclopropyisuifonarnide 42 in 80% overall yield.
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;O'MEARA, Jeffrey;BORDELEAU, Josée;GORYS, Vida;LEBLANC, Mélissa;LENHARDT, Kirsten;LLINAS-BRUNET, Montse;PARISIEN, Mathieu;POUPART, Marc-André WO2010/34105, 2010, A1 Location in patent:Page/Page column 57-58
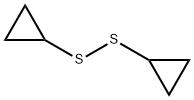
68846-57-1
30 suppliers
inquiry

139631-62-2
252 suppliers
$10.00/1g
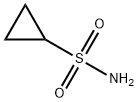
154350-29-5
289 suppliers
$6.00/1g

139631-62-2
252 suppliers
$10.00/1g