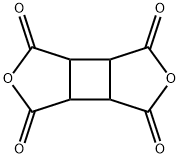
Cyclobutane-1,2,3,4-tetracarboxylic dianhydride synthesis
- Product Name:Cyclobutane-1,2,3,4-tetracarboxylic dianhydride
- CAS Number:4415-87-6
- Molecular formula:C8H4O6
- Molecular Weight:196.11

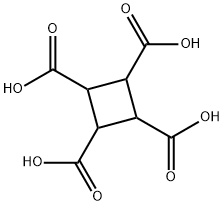
53159-92-5
63 suppliers
$50.00/250mg

4415-87-6
247 suppliers
$6.00/1g
Yield:4415-87-6 92.5%
Reaction Conditions:
with acetic anhydride at 100; for 24 h;Inert atmosphere;
Steps:
1.3
(3) 19.0 g of cyclobutanetetracarboxylic acid obtained in step (2) was added to 190 g of acetic anhydride, stirred and heated to 100 °C for 24 h under nitrogen protection, cooled to 10 °C and filtered, the filter cake was added with 100 mL of acetone and heated to reflux, boiled Washed for 2 hours, cooled to 10°C, filtered, rinsed with acetone, and dried to obtain 14.8 g of cyclobutanetetracarboxylic dianhydride with a yield of 92.5% and a purity of 99.3%.
References:
CN114516882,2022,A Location in patent:Paragraph 0009; 0035-0036; 0039
108-31-6
871 suppliers
$13.47/50g

4415-87-6
247 suppliers
$6.00/1g

108-31-6
871 suppliers
$13.47/50g

74-85-1
110 suppliers
$79.00/11l

4415-87-6
247 suppliers
$6.00/1g
4462-96-8
123 suppliers
$23.00/250mg
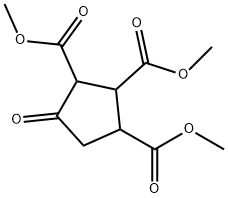
67885-96-5
0 suppliers
inquiry

4415-87-6
247 suppliers
$6.00/1g