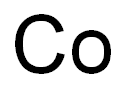
Cobalt synthesis
- Product Name:Cobalt
- CAS Number:7440-48-4
- Molecular formula:Co
- Molecular Weight:58.93
Practically all cobalt produced is a by- or coproduct of other metals, chiefly copper; accordingly, a description of the mining process is omitted. The processes used in extracting cobalt from its ores vary according to the type of ore and locations of the ore deposit.
Arsenical ores are concentrated by hand sorting, gravity separation, or froth flotation, and are smelted in a blast furnace with coke and limestone to a speiss (an impure mixture of iron, cobalt, and nickel arsenides). The speiss is ground, roasted with salt, and leached with water. Insoluble chlorides remaining after the leaching process are ground with sulfuric acid, washed, and filtered, and the washings are added to the liquid from the leaching step. The combined solution is oxidized and then neutralized with lime.
Basic ferric arsenate precipitates and is removed, leaving a solution-containing cobalt and nickel. The addition of successive portions of sodium hydroxide and sodium hypochlorite precipitates cobalt as the hydroxide, which is initially pure but finally admixes with nickel hydroxide. The cobalt precipitate is dried, ground, and formed into pellets, which are reduced by heating with charcoal to cobalt metal.
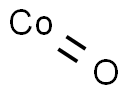
1307-96-6
367 suppliers
$9.00/25g
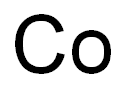
7440-48-4
283 suppliers
$30.00/25g
Yield:-
Reaction Conditions:
with arsenic;sodium hydroxide in meltbyproducts: Na3AsO4, H2O; reduction Of CoO with As in NaOH-melt;;
References:
Kirsebom, G. N. [Tidsskrift for Kjemi og Bergvesen,1940,vol. 20,p. 1 - 7] [Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Co: SVol.A, 22, page 209 - 210]
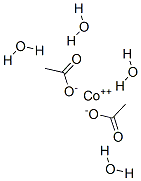
6147-53-1
283 suppliers
$7.00/25g
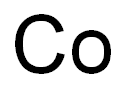
7440-48-4
283 suppliers
$30.00/25g
