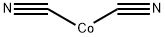
COBALT CYANIDE synthesis
1synthesis methods
- Product Name:COBALT CYANIDE
- CAS Number:542-84-7
- Molecular formula:C2CoN2
- Molecular Weight:110.97
The trihydrate salt is obtained as a reddish brown precipitate by adding potasium cyanide to a cobalt salt solution:
CoCl2 + KCN + 3H2O → Co(CN)2?3H2O + 2KCl
This on dehydration yields anhydrous Co(CN)2. The Co(CN)2?3H2O precipitate formed above redissolves when excess KCN is added, forming a red solution of potassium cobalt(II) cyanide, K4Co(CN)6. Stoichiomtric amount of KCN should, therefore, be used in the preparation of cobalt(II) cyanide.
CoCl2 + KCN + 3H2O → Co(CN)2?3H2O + 2KCl
This on dehydration yields anhydrous Co(CN)2. The Co(CN)2?3H2O precipitate formed above redissolves when excess KCN is added, forming a red solution of potassium cobalt(II) cyanide, K4Co(CN)6. Stoichiomtric amount of KCN should, therefore, be used in the preparation of cobalt(II) cyanide.
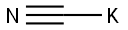
151-50-8
4 suppliers
$43.36/100g
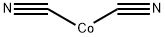
542-84-7
13 suppliers
inquiry
Yield:-
Reaction Conditions:
with water;cobalt(II) chloride
References:
Bigelow [Inorganic Syntheses,1946,vol. 2,p. 226]