Clomiphene Citrate synthesis
- Product Name:Clomiphene Citrate
- CAS Number:50-41-9
- Molecular formula:C32H36ClNO8
- Molecular Weight:598.08
19957-52-9
29 suppliers
$65.00/5mg
77-92-9
1691 suppliers
$5.00/10g

50-41-9
446 suppliers
$15.00/500mg
Yield:50-41-9 361 g
Reaction Conditions:
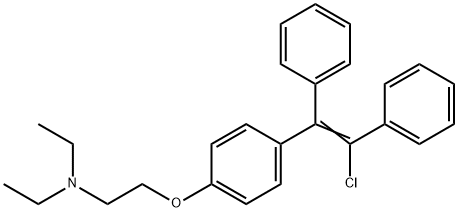
Stage #1: 2-{4-(1,2-diphenylethenyl)phenoxy}-N,N-diethylethanamine hydrochloridewith 1,3-dichloro-5,5-dimethylhydantoin;acetic acid in toluene at 60; for 2 h;
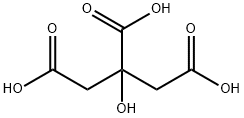
Stage #2: citric acid in acetone at 0; for 4.5 h;Heating;Temperature;
Steps:
1.2; 1.3 Preparation of Clomiphene Citrate from 1-{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethanol
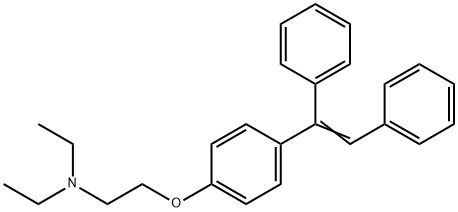
The reaction mixture was brought to 60° C. and, maintaining this temperature, 500 mL of acetic acid were added during 30′. The water content was checked below 500 ppm (Karl Fischer titration).A warm solution (50-60° C.), prepared with 60 g of dichloro dimethyl hydantoin (1,3-dichloro-5,5-dimethyl hydantoin; abbreviated DCDMI) (0.474 mol. equiv.) in 600 mL of toluene, was slowly added to the reaction mixture during 90′ maintaining the temperature at about 60° C. After checking the conversion by HPLC, a small addition of DCDMI solution was necessary in order to have the starting material below 0.5%. The reaction mixture was cooled to 15-20° C. and treated slowly with 750 mL of water and, up to pH 12, approximately 1000 mL of aqueous sodium hydroxide 30% w/w solution. After stirring for 30′, the layers were separated and the organic phase was washed with 3×250 mL of water and concentrated by vacuum distillation thus obtaining 280 g of Clomiphene, as an oil containing some residual toluene of formula (I) and (II).The oil obtained in the previous step was taken up with 625 mL of acetone, the clear solution warmed to gentle boiling and a solution composed of 142.5 g of citric acid monohydrate and 1000 mL of acetone was slowly added during 30′. The reaction mixture was stirred while slowly cooling to 0° C. and then 4 hours at 0° C. The product was filtered, washed with acetone and dried under vacuum obtaining 361 g of Clomiphene citrate, the molar yield was 94.1%. HPLC purity >98% (A/A%).
References:
US2016/152551,2016,A1 Location in patent:Paragraph 0098; 0101; 0102; 0103; 0104; 0105