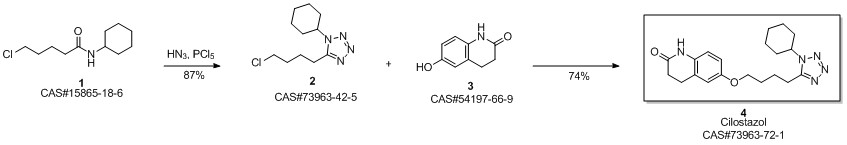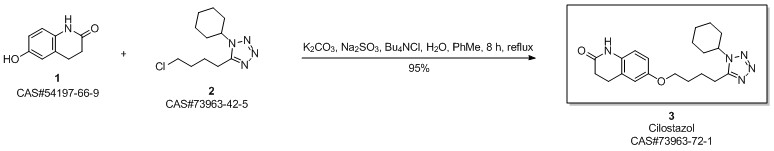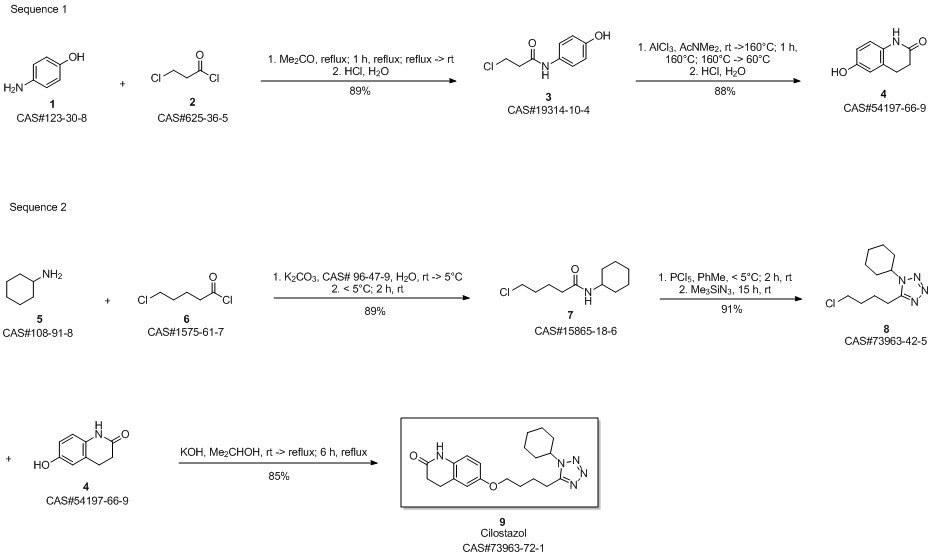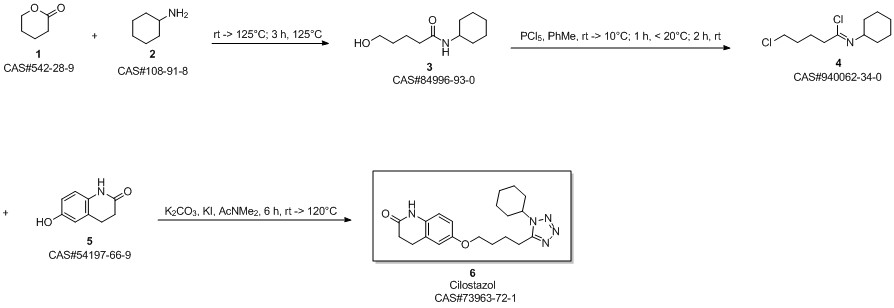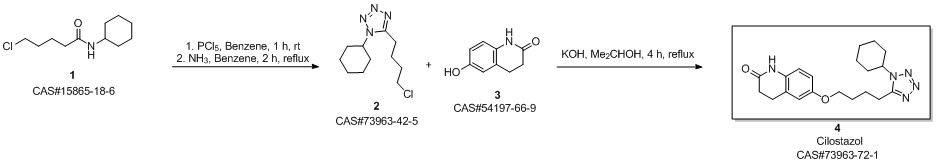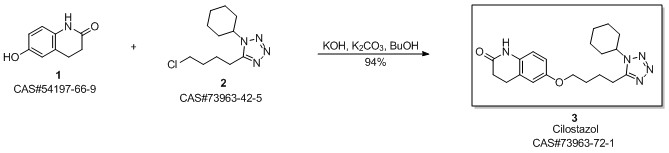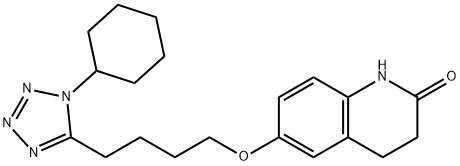
Cilostazol synthesis
- Product Name:Cilostazol
- CAS Number:73963-72-1
- Molecular formula:C20H27N5O2
- Molecular Weight:369.46
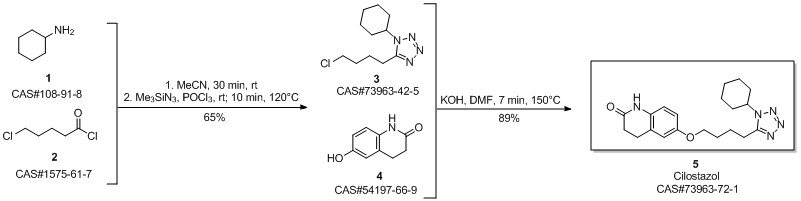
Chandgude, Ajay L.; Doemling, Alexander. Convergent Three-Component Tetrazole Synthesis. European Journal of Organic Chemistry. Department of Drug Design. University of Groningen. Groningen, Neth. 9713 AV. Volume 2016. Issue 14.Pages 2383-2387. 2016
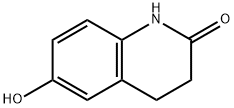
54197-66-9
375 suppliers
$8.00/5g
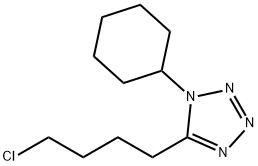
73963-42-5
292 suppliers
$16.00/1g

73963-72-1
461 suppliers
$29.00/500 mg
Yield:73963-72-1 92.5%
Reaction Conditions:
with potassium carbonate;sodium hydroxide;sodium sulfite in ethanol for 8 h;Reflux;
Steps:
6 Example 6
In the 5000L reactor,1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole 296 g (1.22 mol) was added,6-Hydroxy-3,4-dihydroquinolones 180 g (1.1 mol), potassium carbonate 385 g (2.79 mol), sodium hydroxide 36 g (0.9 mol), sodium sulfite 9 g (0.071 mol), 66.7% ethanol 1600 g, kept at reflux 8 Hours, after the completion of the reaction, add 600g of water, stir and reflux for 40min, stand for stratification, transfer the alkaline aqueous phase to the wastewater system, reduce the organic phase to 5 degrees, stir and crystallize, filter, rinse with part of ethanol. It was dried under reduced pressure at 85°C and 377 g of a white solid was obtained with a yield of 92.5% and a purity of 99.8%. Melting point 159 .
References:
Zhejiang Jinliyuan Pharmaceutical Co., Ltd.;Zhang Xiaowei;Sheng Kaiman;Zhao Binfeng;Zhang Xiaofeng CN107382970, 2017, A Location in patent:Paragraph 0023; 0024; 0029; 0030; 0031; 0032; 0033-0040

54197-66-9
375 suppliers
$8.00/5g

73963-42-5
292 suppliers
$16.00/1g
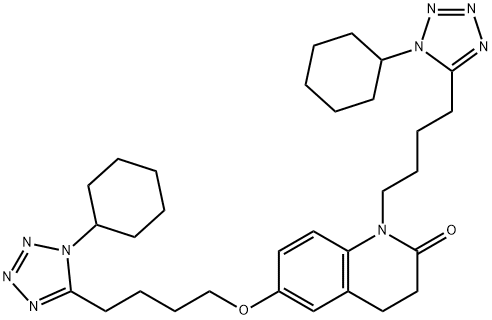
865792-18-3
53 suppliers
$515.00/100mg

73963-72-1
461 suppliers
$29.00/500 mg
![6-[4-(1-CYCLOHEXYL-1H-TETRAZOL-5-YL) BUTOXY]-2(1H)-QUINOLINONE](/CAS/GIF/73963-62-9.gif)
73963-62-9
82 suppliers
$125.00/2 mg

73963-42-5
292 suppliers
$16.00/1g

73963-72-1
461 suppliers
$29.00/500 mg

104-94-9
503 suppliers
$9.00/1g

73963-72-1
461 suppliers
$29.00/500 mg
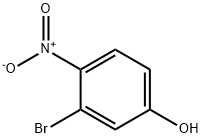
5470-65-5
107 suppliers
$73.00/250mg

73963-72-1
461 suppliers
$29.00/500 mg