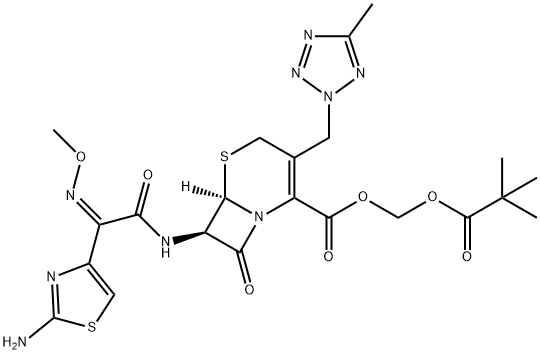
Cefteram pivoxil synthesis
- Product Name:Cefteram pivoxil
- CAS Number:82547-81-7
- Molecular formula:C22H27N9O7S2
- Molecular Weight:593.64
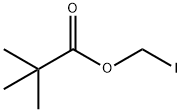
53064-79-2
198 suppliers
$19.00/1g
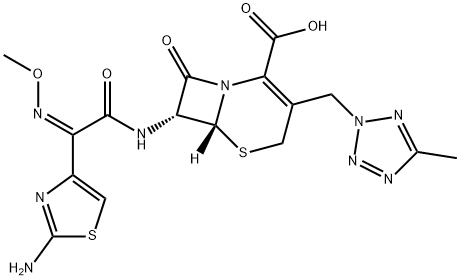
82547-58-8
98 suppliers
$110.00/2mg

82547-81-7
87 suppliers
$60.00/1 mg
Yield:82547-81-7 53.2%
Reaction Conditions:
Stage #1:cefteram with sodium methylate in methanol;N,N-dimethyl-formamide at -20; for 0.333333 h;
Stage #2:iodomethyl pivaloate in methanol;N,N-dimethyl-formamide at -20; for 2.5 h;
Steps:
1.3 3) Preparation of cefteram pivoxil
10.0 g of cefteram acid, 73 mL of DMF was added to the reaction flask, cooled to -20 ° C, 1.2 mL of methanol and 3.75 mL of 30% sodium methoxide were added. The reaction was stirred at -20 ° C. After 20min,33.3 mmol of iodomethyl pivalate were added dropwise, and the temperature was maintained for 2.5 h (the reaction was monitored by HPLC).After the reaction is completed, add 55 mL of ethyl acetate, 55 mL of 8 mol / L hydrochloric acid, stir, and separate the phases; waterThe phases were extracted again with ethyl acetate (50 mL × 2); the organic phase was washed with 175 mL of 1 mol / L hydrochloric acid, and then washed with a mixture of 0.5 g of sodium metabisulfite, 1.6 g of sodium bicarbonate, 15.0 g of sodium chloride and 175 mL of water, 1.0g activated carbon for 30min,Filtration; 10 mL of isopropyl alcohol was added, and the mixed liquid was added dropwise to 300 mL of isopropyl ether, and crystallized for 2.0 hFiltered, washed twice with a small amount of isopropyl ether, and dried. The product was dried under vacuum at 45 ° C to obtain 6.795 g of product with a yield of 53.2% and a purity of 96.2%. .
References:
SHANDONG YUXIN PHARMACEUTICAL CO LTD;Shandong Yuxin Pharmaceutical Co., Ltd.;LIU ZHENTENG;Liu Zhenteng;DONG XUEJU;Dong Xueju;SHENG ZHONGLI;Sheng Zhongli;TIAN SONG;Tian Song CN108299470, 2018, A Location in patent:Paragraph 0048; 0053-0054
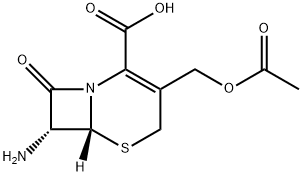
957-68-6
446 suppliers
$14.00/5g

82547-81-7
87 suppliers
$60.00/1 mg