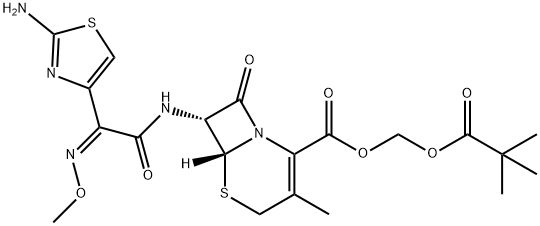
Cefetamet pivoxyl synthesis
- Product Name:Cefetamet pivoxyl
- CAS Number:65243-33-6
- Molecular formula:C20H25N5O7S2
- Molecular Weight:511.57
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
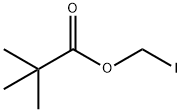
53064-79-2
198 suppliers
$19.00/1g

65243-33-6
73 suppliers
$135.00/10g
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in N,N-dimethyl-formamide for 2 h;
Steps:
1
Sodium 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido]-3- methyl- ceph-3-em-4-carboxylic acid (Cefetamet sodium) To a mixture of water (250 rnL) and acetone (500 mL), 7-Amino desacetoxy cephalosporanic acid (50 g) and 2-mercaptobenzothiazolyl (Z)-2-(2- aminothiazol-4-yl)-2-methoxyimino acetate (90 g) (MAEM) were added at 5- 100C. It was followed by addition of sodium 2-ethylhexanoate (77.0 g) in one lot and stirred till completion of reaction. After completion of reaction, further acetone was added (3L) and the precipitated solid was filtered under nitrogen atmosphere and washed with acetone (500 mL) and dried to give 95 g of Cefetamet sodium of 99 % purity.The cefetamet sodium thus obtained is converted to Cefetamet pivoxil as follows; Preparation of Cefetamet PivoxilTo DMF (25OmL), Cefetamet Sodium (10Og) was added at room temperature and stirred to get clear solution. To the clear solution, solid sodium bicarbonate (1.6g) was added. The reaction mass was cooled to -25 to -300C and iodomethyl pivalate (45.83g) was added in one lot and stirred for 2 h. The reaction mixture was poured into a mixture of ethyl acetate (50OmL) and water (50OmL). Sodium thiosulphate and EDTA were added into this and the mixture was stirred followed by pH adjustment with the dilute HCl. The layers were separated and the organic layer was washed with brine solution. The organic layer was charcolised and filtered, the carbon bed was washed with ethyl acetate. The ethyl acetate layer was poured into isopropyl ether (2.5L). The solid obtained
References:
ORCHID CHEMICALS & PHARMACEUTICALS LIMITED;UDAYAMPALAYAM, Palanisamy, Senthilkumar;SIVAKUMARAN, Sundaravadivelan;SAHOO, Prabhat, Kumar WO2008/41100, 2008, A1 Location in patent:Page/Page column 9-10