Capsaicin synthesis
- Product Name:Capsaicin
- CAS Number:404-86-4
- Molecular formula:C18H27NO3
- Molecular Weight:305.41
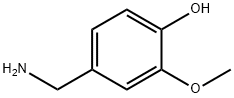
1196-92-5
42 suppliers
$544.00/500g
59320-77-3
88 suppliers
$28.00/10mg

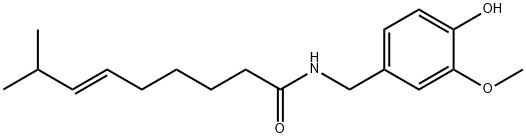
404-86-4
669 suppliers
$28.00/100mg
Yield:404-86-4 99.7 %
Reaction Conditions:
with Novozym 435 in di-isopropyl ether at 69;Dean-Stark;Solvent;Temperature;Reagent/catalyst;
Steps:
11; 15; 17; 22-25 Example 11: Preparation of capsaicin with excess amine in DIPE.
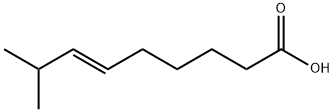
At normal atmospheric pressure (approximately 1 atm), 8-methyl-6-nonenoic acid (3.65 g), vanillyl amine (1.1 equiv.), Novozym 435(tm) on beads (498.8 mg, 14 w/w% E/S) were refluxed (about 69 oC) in diisopropyl ether (45 mL) with a Dean-Stark trap to collect the generated water. After stirring at about 300 rpm overnight (19 h), the mixture was filtered to recover the lipase catalyst, and the filtrate was washed with 0.5 M aqueous HCl solution (10 mL). The aqueous phase was extracted with Et2O (10 mL×2) and the organic phase were combined, dried over anhydrous Na2SO4 and concentrated to give 6.53 g product ( % yield, very pale yellow color).
References:
WO2022/229314,2022,A1 Location in patent:Page/Page column 25-26; 28-31

59320-77-3
88 suppliers
$28.00/10mg
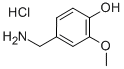
7149-10-2
312 suppliers
$6.00/5g

404-86-4
669 suppliers
$28.00/100mg

1196-92-5
42 suppliers
$544.00/500g
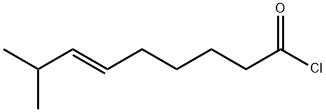
95636-02-5
62 suppliers
$133.00/1g

404-86-4
669 suppliers
$28.00/100mg

1196-92-5
42 suppliers
$544.00/500g
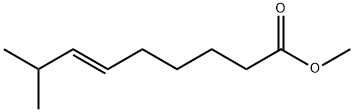
112375-54-9
8 suppliers
inquiry

404-86-4
669 suppliers
$28.00/100mg

59320-77-3
88 suppliers
$28.00/10mg

404-86-4
669 suppliers
$28.00/100mg
