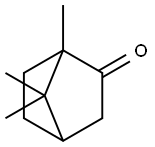
Camphor synthesis
- Product Name:Camphor
- CAS Number:76-22-2
- Molecular formula:C10H16O
- Molecular Weight:152.23
![(±)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime](/CAS/20180808/GIF/18674-50-5.gif)
18674-50-5
1 suppliers
inquiry

76-22-2
601 suppliers
$10.00/100g
Yield:76-22-2 83%
Reaction Conditions:
with water;oxygen in acetonitrile at 60; under 760.051 Torr; for 6 h;Autoclave;Green chemistry;
Steps:
General experimental procedure for deoximation to the corresponding carbonyl compounds
General procedure: Oximes (1.0 mmol), Amberlyst-15 (0.02 g), FPA53-NO2(0.02 g), and solvent (1.5 mL) were introduced into a 25-cm-high, 90-mL autoclave with a glass tube inside equipped with magnetic stirrer (Scheme 2). Then the autoclave was charged with oxygen to 0.1 MPa. The reaction mixture was stirred at desirable temperature for special time. Progress of the reaction was monitored by thin-layer chromatography (TLC) or gas chromatography (GC). After the reaction, the resin (Amberlyst-15 and FPA53-NO2) was separated from the reaction mixture by filtration and extracted with 3 mL CH3CN (2 1.5 ml). The solvent was removed under reduced pressure. The residue was further purified by column chromatography on silica gel (300 mesh) with hexane/ethyl acetate to give the corresponding carbonyl compounds.
References:
Guo, Shu;Zeng, Renyou;Li, Caiye [Synthetic Communications,2016,vol. 46,# 17,p. 1446 - 1453] Location in patent:supporting information
24393-70-2
0 suppliers
inquiry

76-22-2
601 suppliers
$10.00/100g
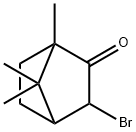
76-29-9
30 suppliers
$121.00/100mg

76-22-2
601 suppliers
$10.00/100g
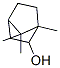
6627-72-1
76 suppliers
inquiry

76-22-2
601 suppliers
$10.00/100g
![Spiro[bicyclo[2.2.1]heptane-2,2'-[1,3]dithiolane], 1,7,7-trimethyl-](/CAS/20180531/GIF/6787-91-3.gif)
6787-91-3
0 suppliers
inquiry

76-22-2
601 suppliers
$10.00/100g