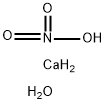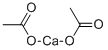
Calcium acetate synthesis
- Product Name:Calcium acetate
- CAS Number:62-54-4
- Molecular formula:C4H6CaO4
- Molecular Weight:158.17
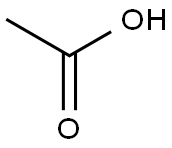
64-19-7
1585 suppliers
$10.00/25ML
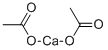
62-54-4
466 suppliers
$5.00/25g
Yield:62-54-4 100%
Reaction Conditions:
with calcium carbonate in water
Steps:
4.1.1. Calcium acetate monohydrate, Ca(OAc)2·H2O
Calcium acetate was prepared from calcium carbonate and dilutedacetic acid. The solubility of calcium acetate decreases with temperature,thus no crystalline material can be obtained by cooling ofsaturated solutions [36]. Calcium acetate monohydrate was insteadisolated by salting out with acetone from the reaction mixture,following the procedure described by Panzer [29]. Acetone was slowlyadded to the vigorously stirred solution to form a fine precipitate.When no more solid formed the precipitate was filtered off withsuction. The filter cake was washed with copious amounts of acetone,isolated and suspended again in acetone with vigorous stirring, and thesolid again filtered off with suction. The process was repeated twiceuntil the filter cake was essentially odorless (no acetic acid smell). Thesolid was dried in air for several days with intermediate breaking upand grinding, and analyzed by powder X-ray diffraction. Rietveldrefinement yielded one of the triclinic polymorphs of calcium acetatemonohydrate [16] with lattice constants a = 6.755, b = 11.103, c =11.801 ?, α = 116.41, β = 92.40, γ = 97.52° as the only crystallinecomponent, with no indication of the other reported polymorph[37,38] being present (see SI for details, Fig. S1). The material wasstored in a closed container until further use. The yield was essentiallyquantitative.
References:
Miller, Jennifer R.;LaLama, Matthew J.;Kusnic, Rachel L.;Wilson, Darian E.;Kiraly, Paije M.;Dickson, Samuel W.;Zeller, Matthias [Journal of Solid State Chemistry,2019,vol. 270,p. 1 - 10]
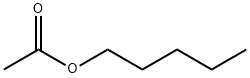
628-63-7
400 suppliers
$18.00/25mL
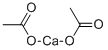
62-54-4
466 suppliers
$5.00/25g
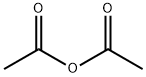
108-24-7
0 suppliers
$14.00/250ML
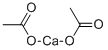
62-54-4
466 suppliers
$5.00/25g