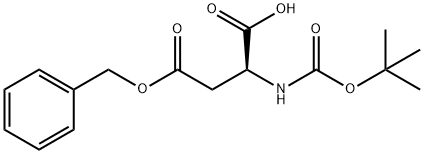
Boc-L-aspartic acid 4-benzyl ester synthesis
- Product Name:Boc-L-aspartic acid 4-benzyl ester
- CAS Number:7536-58-5
- Molecular formula:C16H21NO6
- Molecular Weight:323.34

24424-99-5
861 suppliers
$13.50/25G
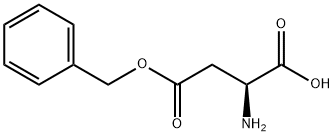
2177-63-1
286 suppliers
$6.00/5g

7536-58-5
330 suppliers
$6.00/5g
Yield:7536-58-5 90%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran at 0 - 20; for 12.5 h;
Steps:
4.1 Boc-L-aspartic acid 4-benzyl ester
In the single-port in round bottomed flask add L - aspartic acid -4 - benzyl ester (79.7g, 357mmol), sodium bicarbonate (60g, 714.2mmol), water (700 ml) and tetrahydrofuran (550 ml). The reaction system is cooled to 0 °C, the reaction bottle adds by drops two carbon acid tert-butyl ester in (105g, 481mmol), the completion of the dropping, 0 °C reaction under half an hour later, slowly increased to room temperature, stirring for 12 hours. Reduced pressure to remove tetrahydrofuran, aqueous phase of ethyl acetate (20 ml) washing. The aqueous phase is 10% of the aqueous solution of citric acid (10 ml) to adjust the pH to 1, ethyl acetate (200 ml × 2) extraction, the combined organic phase, the organic phase with saturated salt water (100 ml × 2) washing, drying with anhydrous sodium sulfate. Filtering, evaporating the solvent under reduced pressure, to obtain white solid (104g, 90%).
References:
Guangdong Dongyangguang Pharmaceutical Co., Ltd.;Dongguan Dongyangguang Pharmaceutical Research And Development Co., Ltd.;Zhou Pingjian;Yang Chuanwen;Lin Jihua;Wang Xiaojun;Lao Jinhua;Xue Yaping;Cao Shengtian;Wu Fangyuan;Zhang Yingjun CN106866502, 2017, A Location in patent:Paragraph 0230-0234
13726-67-5
287 suppliers
$6.00/5g
100-51-6
1431 suppliers
$5.00/100g

7536-58-5
330 suppliers
$6.00/5g
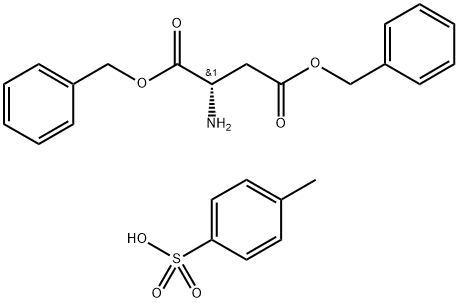
2886-33-1
180 suppliers
$8.00/5g

7536-58-5
330 suppliers
$6.00/5g

100-51-6
1431 suppliers
$5.00/100g

7536-58-5
330 suppliers
$6.00/5g
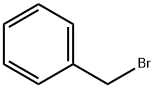
100-39-0
438 suppliers
$10.00/10 g

7536-58-5
330 suppliers
$6.00/5g