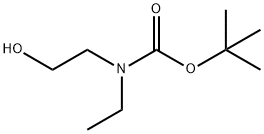
BOC,ET-N-ET-OH synthesis
- Product Name:BOC,ET-N-ET-OH
- CAS Number:152192-95-5
- Molecular formula:C9H19NO3
- Molecular Weight:189.25
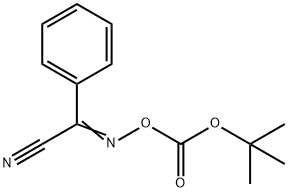
58632-95-4
268 suppliers
$7.00/5g
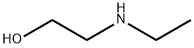
110-73-6
280 suppliers
$5.00/5g

152192-95-5
65 suppliers
$26.00/1g
Yield:-
Reaction Conditions:
with triethylamine in 1,4-dioxane;water
Steps:
29.A Step A
Step A 2-(N-Tert-butyloxycarbonyl-N-ethyl)aminoethanol To a solution of 2-(ethylamino)ethanol (3 g, 33.65 mmol) in 1:1 dioxane:water at 0° C. was added triethylamine(7.04 ml, 50.48 mmol) followed by addition of 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile (9.9 g, 40.38 mmol). The reaction mixture was stirred at 0° C. for 10 min and then at room temperature for 2 h. The mixture was extracted with ethyl acetate (2*150 ml); the ethyl acetate layers were then combined, washed with brine and dried over sodium sulfate. Removal of the solvent and subsequent purification by column chromatography eluding with 1:2 ethyl acetate:hexane system to obtain 3.69 g of the title compound. 1H NMR (500 MHz, CDCl3): δ3.753 (t, J=5.0 Hz, 2H); 3.39 (t, J=4.8 Hz, 2H); 3.24 (brs, 2H); 1.48 (s, 9H); 1.13 (t, J=7.1 Hz, 3H).
References:
Merck & Co., Inc. US6316444, 2001, B1

24424-99-5
840 suppliers
$13.50/25G
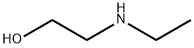
110-73-6
280 suppliers
$5.00/5g

152192-95-5
65 suppliers
$26.00/1g

24424-99-5
840 suppliers
$13.50/25G
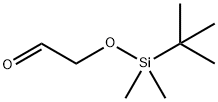
102191-92-4
147 suppliers
$10.00/250mg
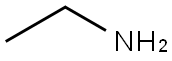
75-04-7
396 suppliers
$10.00/20mg

152192-95-5
65 suppliers
$26.00/1g