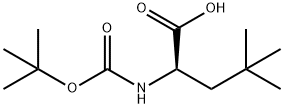
BOC-BETA-TBU-D-ALA-OH synthesis
- Product Name:BOC-BETA-TBU-D-ALA-OH
- CAS Number:112695-98-4
- Molecular formula:C12H23NO4
- Molecular Weight:245.32

24424-99-5
863 suppliers
$13.50/25G
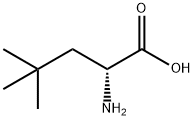
88319-43-1
104 suppliers
$45.00/10mg

112695-98-4
83 suppliers
$60.50/50mg
Yield:112695-98-4 99.5%
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;(R)-2-amino-4,4-dimethylpentanoic acidwith sodium hydroxide in tetrahydrofuran;water at 20;
Stage #2: with sodium hydrogen sulfate in tetrahydrofuran;water;
Steps:
5.a a)
To a solution of (R)-2-amino-4,4-dimethyl-pentanoic acid (1.452 g, 10 mmol) in water (10 mL) was added NaOH (0.44 g, 11 mmol) and a solution of Boc-anhydride (2.292 g, 10.5 mmol) in THF (10 mL). The cloudy mixture, which gradually became clear and then cloudy again, was stirred at room temperature over night. Most of the THF was evaporated and the residue was acidified with 1M NaHSO4 and extracted (3*) with DCM. The combined organic phases were dried, filtered and evaporated to give the pure product (2.44 g, 99.5%).
References:
US2009/12087,2009,A1 Location in patent:Page/Page column 15, 16
2987-16-8
214 suppliers
$5.00/1g

112695-98-4
83 suppliers
$60.50/50mg
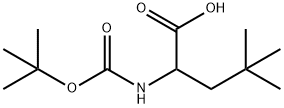
507264-54-2
21 suppliers
$165.00/1g

112695-98-4
83 suppliers
$60.50/50mg