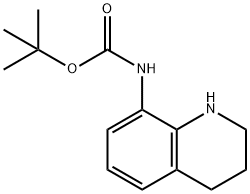
BOC-8-AMINO-1,2,3,4-TETRAHYDROQUINOLINE synthesis
- Product Name:BOC-8-AMINO-1,2,3,4-TETRAHYDROQUINOLINE
- CAS Number:137469-86-4
- Molecular formula:C14H20N2O2
- Molecular Weight:248.32
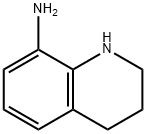
54012-92-9
32 suppliers
inquiry

24424-99-5
848 suppliers
$13.50/25G

137469-86-4
11 suppliers
inquiry
Yield:137469-86-4 75.26%
Reaction Conditions:
in tetrahydrofuran at 0; for 3 h;
Steps:
Tert-butyl (1,2,3,4-tetrahydroquinolin-8-yl)carbamate (M3)
Intermediate M2 (0.96 g, 6.48 mmol) was dissolved in 10 mL tetrahydrofuran and cooled to 0 °C. Then, 1.41 g (6.48 mmol) di-tert-butyl pyrocarbonate were dissolved in 5 mL of tetrahydrofuran and dripped into the reaction system within one hour. The mixture was stirred at 0 °C for another two hours, and then, the solvent was evaporated under reduced pressure. The residue was purified on a silica gel column (petroleum ether: ethyl acetate = 10: 1) to obtain M3 (1.21 g, 75.26 %) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 8.01 (s, 1H), 6.97 (d, J = 7.6 Hz, 1H), 6.67 (d, J = 7.4 Hz, 1H), 6.41 (t, J = 7.6 Hz, 1H), 4.99 (s, 1H), 3.20 (td, J = 5.9, 3.1 Hz, 2H), 2.67 (t, J = 6.3 Hz, 2H), 1.80 - 1.71 (m, 2H), 1.44 (s, 9H).
References:
Cheng, Maosheng;Jiang, Qinwen;Wang, Jiming;Wang, Xinran;Yan, Jiangkun;Zhang, Cai;Zhang, Xiangyu;Zhao, Dongmei;Zhao, Liyu [European Journal of Medicinal Chemistry,2020,vol. 194] Location in patent:supporting information
578-66-5
324 suppliers
$6.00/1g

137469-86-4
11 suppliers
inquiry
137469-85-3
1 suppliers
inquiry

137469-86-4
11 suppliers
inquiry

24424-99-5
848 suppliers
$13.50/25G

137469-86-4
11 suppliers
inquiry
607-35-2
259 suppliers
$12.00/1mg

137469-86-4
11 suppliers
inquiry