Bisphenol A synthesis
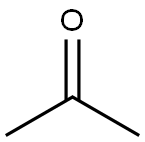
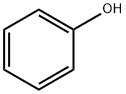
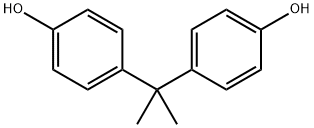
- Product Name:Bisphenol A
- CAS Number:80-05-7
- Molecular formula:C15H16O2
- Molecular Weight:228.29
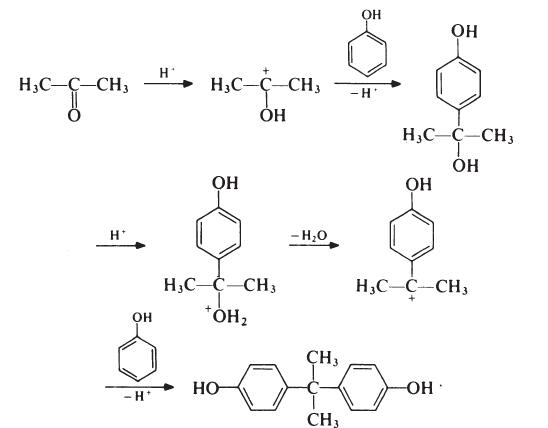
Although the reaction theoretically requires the molar ratio of reactants to be 2: 1, an improved yield of bisphenol A is obtained if additional phenol is present; the optimum molar ratio is 4: 1. In a typical process, the phenol and acetone are mixed and warmed to 50°C. Hydrogen chloride (catalyst) is passed into the mixture for about 8 hours, during which period the temperature is kept below 70°C to suppress the formation of isomeric products. Bisphenol A precipitates and is filtered off and washed with toluene to remove unreacted phenol (which is recovered). The product is then recrystallized from aqueous ethanol. Since epoxy resins are oflow molecular weight and because colour is not normally particularly important, the purity of bisphenol A used in resin production is not critical. Material with a p,p'-isomer content of 95-98% is usually satisfactory; the principal impurities in such material are o,p'- and o,o'-isomers.

79-94-7
433 suppliers
$30.00/25g

80-05-7
693 suppliers
$13.00/100g
Yield:80-05-7 95%
Reaction Conditions:
with hydrogen;triethylamine in ethanol;water at 120; under 22502.3 Torr; for 116 h;Autoclave;
Steps:
4.3 General Procedure for the Preparation of substituted Anilines from Nitroarenes
General procedure: In a 4 ml_ reaction glass vial fitted with a septum cap containing a magnetic stirring bar, Co-Co3O4Chit-700 (10 mg, 3.4 mol% Co), the nitroarenes (0.5 mmol, 1 .0 equiv.) and triethylamine (35 μΙ_, 0.25 mmol, 0.5 equiv.) were added to a solvent mixture of EtOH/H20 (3/1 , 2 ml_). The reaction vial was then placed into a 300 ml_ autoclave, flashed with hydrogen five times and finally pressurized to 40 bar. The reaction mixture was stirred for appropriate time at 1 10 °C. After cooling the reaction mixture to room temperature, the autoclave was slowly depressurized. The crude reaction mixture was filtered through a pipette fitted with a cotton bed and the solvent was evaporated under reduced pressure. The crude products were purified by passing through a silica plug (eluent: ethyl acetate) to give pure aniline derivatives after removal of solvent. The following compounds may be prepared from the respective nitroarenes using the catalyst of the invention:
References:
WO2018/114777,2018,A1 Location in patent:Page/Page column 31; 37
4387-16-0
23 suppliers
inquiry

80-05-7
693 suppliers
$13.00/100g