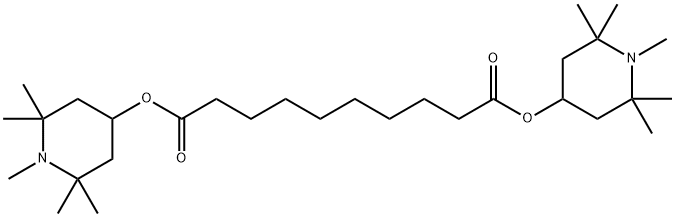
Bis(1,2,2,6,6-pentamethyl-4-piperidyl) sebacate synthesis
- Product Name:Bis(1,2,2,6,6-pentamethyl-4-piperidyl) sebacate
- CAS Number:41556-26-7
- Molecular formula:C30H56N2O4
- Molecular Weight:508.78
50-00-0
892 suppliers
$10.00/25g
52829-07-9
390 suppliers
$5.00/25g

41556-26-7
315 suppliers
$10.00/10g
Yield:-
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol;water at 80 - 140; under 15001.5 - 31503.2 Torr;Autoclave;Solvent;
Steps:
I.14 Inventive Examples I11-I14
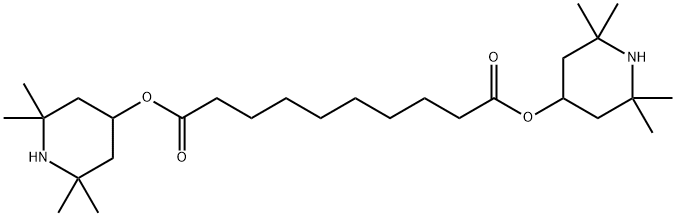
General procedure: Inventive Examples I11-I14 (0562) A 1 l pressure autoclave was charged with the amount of the particular triacetonamine compound specified in Table 2 (“reactant” according to Table 2), 0.2 mol % of Pd (mol % based on the amount of the reactant) as Pd/activated carbon catalyst, and 250 ml of solvent (methanol or toluene). Thereafter, paraformaldehyde was added in the amount according to Table 2 and rinsed in with 50 ml of solvent, and the reactor was closed. Hydrogen was injected while stirring (40 bar H2). Hydrogenation was effected at a temperature of 80 to 140° C. and a pressure of 20 to 42 bar until no significant uptake of hydrogen was observed any longer. The reactor was then cooled and decompressed. The crude product was discharged and filtered, and then the solvent was first removed (80-120° C., standard pressure). Then the yield of product in the crude product obtained was determined by gas chromatography (=GC, Agilent 5890 or 7890, FID detector) in the case of Examples I11, I12, I14, and by means of MS-ESI in the case of Example I13 (see Table 2, structures in the “crude product” column). In the case of Example I11, the residue was then purified as far as possible by means of a vacuum distillation. (0563) The results are compiled in Table 2.
References:
Evonik Degussa GmbH;NIEMEYER, Jochen;WILLY, Benjamin;NISSEN, Felix;NEUMANN, Manfred;KREILKAMP, Guenter;SCHERING, Sabine US2016/214962, 2016, A1 Location in patent:Paragraph 0562; 0563
