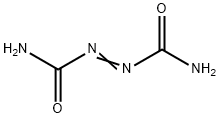
Azodicarbonamide synthesis
- Product Name:Azodicarbonamide
- CAS Number:123-77-3
- Molecular formula:C2H4N4O2
- Molecular Weight:116.08
Yield:-
Reaction Conditions:
with hydrogenchloride in sodium hydroxide;water;dimethyl sulfoxide;
Steps:
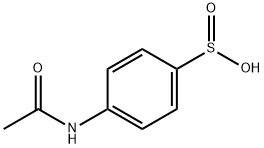
1 Reaction of 1,1' azobisformamide with p-acetamidobenzenesulfinic Acid
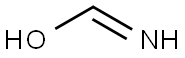
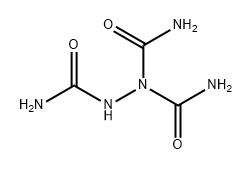
EXAMPLE 1 Reaction of 1,1' azobisformamide with p-acetamidobenzenesulfinic Acid Solutions of 1,1' azobisformamide (2.32 g., 0.02 mol) in 50 ml of dimethyl sulfoxide and p-acetamidobenzenesulfinic acid (3.98 g., 0.02 mol) in 25 ml of dimethyl sulfoxide are combined. After several hours at room temperature, the clear, pale orange reaction mixture is added to 300 ml of water. The resulting clear, pale yellow solution is cooled to ice bath temperatures and after about 0.5 hour the mixture becomes a solid gelatinous mass. The reaction mixture is filtered in a solid filter-cake and washed consecutively with water, alcohol, and ether to give 5.5 g. of a white solid, identified as l,p-acetamidobenzenesulfonyl-1,2-bis(formamoyl) hydrazine. A portion (1.5 g.) of the dried reaction product (5.5 g.) is dissolved in aqueous 10 percent sodium hydroxide. Neutralization of the filtered, cleared alkaline solution with diluted 3N hydrochloric acid gives a crystalline solid, l,p-acetamidobenzenesulfonyl semicarbazide.
References:
US3933909,1976,A

123-77-3
297 suppliers
$12.00/10g
106-99-0
260 suppliers
$80.00/100mL
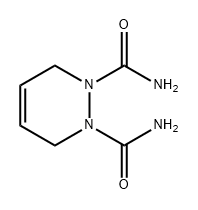
17644-85-8
0 suppliers
inquiry
![N-{4-[(carbamoylamino)sulfamoyl]phenyl}acetamide](/CAS/20200119/GIF/10396-14-2.gif)