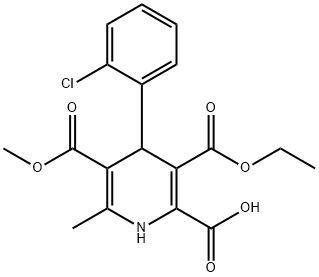
Amlodipine Impurity 28 synthesis
- Product Name:Amlodipine Impurity 28
- CAS Number:79781-21-8
- Molecular formula:C18H20ClNO5
- Molecular Weight:365.81
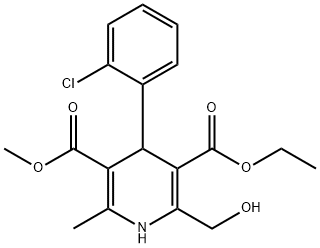
144686-65-7
1 suppliers
inquiry

79781-21-8
15 suppliers
inquiry
Yield:79781-21-8 65%
Reaction Conditions:
with ammonia in methanol at 0; for 2 h;
Steps:
11.E
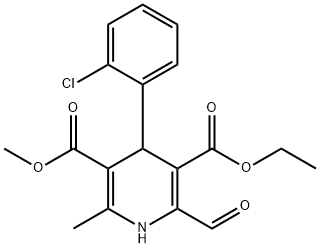
Intermediate E: 3-Ethyl 5-methyl 4-(2-chlorophenyl)-2-(hydroxymethyl)-6-methyl-1 ,4- dihydropyridine-3,5-dicarboxylate. To a solution of intermediate D (1 .2 g, 2.9 mmol, 1 .0 eq) in methanol (20 mL) was added a methanolic ammonia solution (1 .0 M, 15 mL, 15 mmol). The mixture was stirred at 0 °C for 2 h then the solvent was removed under reduced pressure and the residue was diluted with water (50 mL). The aqueous mixture was extracted with CH2CI2 (50 mL x 3) and the combined organic layers were washed with brine (60 mL x 2), dried over MgS04 and concentrated under reduced pressure. The residue was purified by flash chromatography (Pet. ether/EtOAc, 1 /10 to 1 /2, v/v) to give 3-ethyl 5-methyl 4-(2-chlorophenyl)-2-(hydroxymethyl)-6-methyl-1 ,4- dihydropyridine-3,5-dicarboxylate (0.70 g, 65%) as a yellow solid.LC-MS (Agilent): Rt 3.28 min; m/z calculated for Ci8H20CINO5 [M+H]+ 366.1 , [M+Na]+ 388.1 , found [M+H]+ 366.1 , [M+Na]+ 388.1 .1H NMR: (400 MHz, CDCI3) δ (ppm): 7.39 (m, 1 H), 7.25 (m, 1 H), 7.13 (m, 1 H), 7.05 (m, 1 H), 5.41 (s, 1 H), 4.75 (d, J = 4.4 Hz, 2H), 4.06 (m, 2H), 3.63 (s, 3H), 2.33 (s, 3H), 1 .20 (t, J = 7.2 Hz, 3H).
References:
WO2012/63085,2012,A2 Location in patent:Page/Page column 82
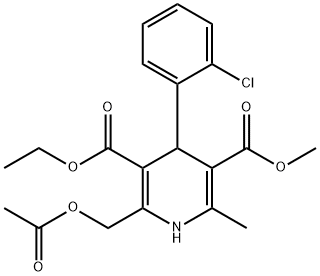
79781-18-3
6 suppliers
inquiry

79781-21-8
15 suppliers
inquiry
129207-93-8
1 suppliers
inquiry

79781-21-8
15 suppliers
inquiry
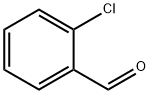
89-98-5
661 suppliers
$16.00/25g

79781-21-8
15 suppliers
inquiry
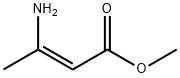
21731-17-9
64 suppliers
inquiry

79781-21-8
15 suppliers
inquiry