alpha-Dihydroartemisinin synthesis
- Product Name:alpha-Dihydroartemisinin
- CAS Number:81496-81-3
- Molecular formula:C15H24O5
- Molecular Weight:284.35
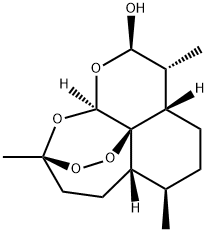
63968-64-9
642 suppliers
$5.00/250mg

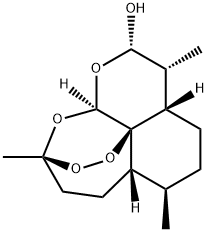
81496-81-3
125 suppliers
inquiry
Yield: 99.6%
Reaction Conditions:
with sodium tetrahydroborate;N-benzyl-N,N,N-triethylammonium chloride in water;toluene at -10 - 0; for 5 h;Inert atmosphere;Large scale;Solvent;
Steps:
1.S1-1.S4; 2.S1-2.S4; 3.S1-3.S4; 4.S1-4.S4; 5.S1-5.S4; 6.S1-6.S4 Example 1A single process for preparing a dihydroartemisinin bulk drug, comprising the steps of:
S1. Dissolving 100 kg of artemisinin in 600 L of toluene;S2. Add 50L of an aqueous solution containing 0.5 kg of benzyltriethylammonium chloride, and cool down to -10 °C.Under the protection of nitrogen, the sodium borohydride reducing agent was added in batches, a total of 16kg.The control temperature does not exceed 0 ° C, the reaction is continued for 5 hours after the addition, and is monitored by thin layer chromatography.When the artemisinin spot completely disappears, the reaction is considered complete;S3. Adjusting the pH of the reaction system obtained in step S2 with a 25% by mass acetic acid solutionValue to 7, the temperature of the control system during the adjustment process is 0-5 ° C;The process is completed in an automatic interlock that includes a temperature sensor, a pH sensor, a sample introduction controller, a stirring device, a central controller, and a control panel.The temperature sensor, the pH sensor, the injection controller, the stirring device,Electrical connection between the central controller and the control panel;The temperature sensor can monitor the temperature in the reaction system and control the temperature to 0-5 ° C through a central controller; the pH sensor is used to monitor the pH change in the reaction system;And the pH value is controlled to about 7 by the central controller; the injection controller is used for controlling the injection amount of the acid liquid, and adjusting the injection speed in time; the stirring device is used for stirring the reaction system to ensure the entire reaction. The system is uniformly neutralized and quenched;After adding water and stirring, the liquid phase was separated, and the aqueous phase obtained by liquid separation was extracted with 200 L of toluene.The organic phase extracted is combined with the liquid organic phase and washed with water.Then adding a sodium sulfate desiccant equivalent to 1% by mass to the organic phase after washing;S4. The organic phase after drying in S3 is pumped into a crystallization-pressure filtration-drying three-in-one crystallization apparatus.Concentrated under reduced pressure at 50 ° C until no solvent is discharged.Add water equal to 5 times the crystal mass, crystallize at 0 ° C, press filter,The pressure of the pressure filtration is 5kg, the filtration time is 30min; finally, it is vacuum dried at 60 °C for 3 hours, which is a dihydroartemisinin product.In this embodiment, the purity of the dihydroartemisinin product is 99.8%, and the yield is 99.6%.750 L of toluene was recovered.
References:
Zhangjiagang Weisheng Bio-pharmaceutical Co., Ltd.;Peng Xuedong;Zhang Mei;Zhao Jinzhao;Yan Yongyi CN110041343, 2019, A Location in patent:Paragraph 0046-0088

63968-64-9
642 suppliers
$5.00/250mg
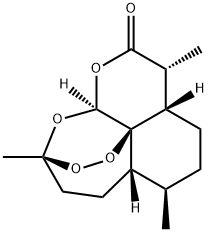
71939-50-9
402 suppliers
$5.00/50mg

81496-81-3
125 suppliers
inquiry

71939-50-9
402 suppliers
$5.00/50mg

81496-81-3
125 suppliers
inquiry