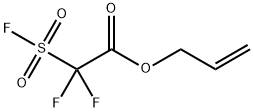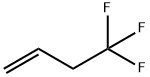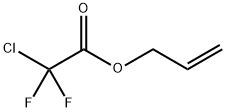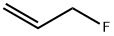
ALLYL FLUORIDE synthesis
- Product Name:ALLYL FLUORIDE
- CAS Number:818-92-8
- Molecular formula:C3H5F
- Molecular Weight:60.07
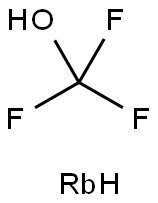
2700-81-4
0 suppliers
inquiry
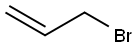
106-95-6
431 suppliers
$10.00/5g
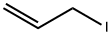
556-56-9
142 suppliers
$10.00/1g
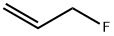
818-92-8
18 suppliers
inquiry
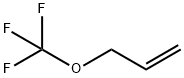
140899-21-4
0 suppliers
inquiry
Yield:140899-21-4 61%
Reaction Conditions:
rubidium iodide in N,N-dimethyl-formamide at 60; for 61 h;
Steps:
6
21.1 g (202 mmol) of dry rubidium fluoride are suspended in 100 ml of dry dimethylformamide (DMF) in a 250 ml round-bottomed flask with reflux condenser (cooled to -78° C.), and 47.9 g (220 mmol) of trifluoromethyl triflate, CF3SO2OCF3, are slowly added with stirring and cooling of the reaction mixture by means of a bath cooled to -45° C. The reaction mixture is kept at -25° C. for 2.5 hours, at 0° C. for 1 hour and at 20° C. for 1 hour. The temperature of the reflux condenser is then increased to -20° C. in order to remove the trifluoromethylsulfonyl fluoride, CF3SO2F, which is formed during the reaction. 4.2 g (20 mmol) of rubidium iodide and 24.2 g (200 mmol) of allyl bromide are added to the suspension of RbOCF3 which remains, and the reaction mixture is stirred at 60° C. for 61 hours. All volatile products are then distilled off in vacuo at 10-2 mbar and room temperature into a cooled distillation trap (-196° C.). In total, 29.7 g of a liquid material which comprises allyl-OCF3, allyl-F, allyl-Br and allyl-I are obtained. This mixture is subjected to fractional distillation, and 15.4 g of allyl-OCF3 are obtained. The yield of allyl trifluoromethyl ether is 61%. The product is characterised by means of 1H- and 19F-NMR spectra. The NMR data for the product, allyl trifluoromethyl ether, are identical to the data described in Example 1.
References:
MERCK PATENT GESELLSCHAFT MIT DESCHRANKTER HAFTUNG US2011/82312, 2011, A1 Location in patent:Page/Page column 6

106-95-6
431 suppliers
$10.00/5g
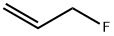
818-92-8
18 suppliers
inquiry
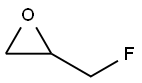
503-09-3
75 suppliers
$40.00/1 g
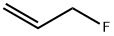
818-92-8
18 suppliers
inquiry