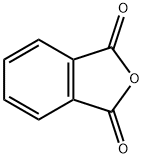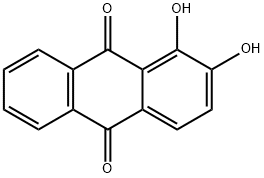
Alizarin synthesis
- Product Name:Alizarin
- CAS Number:72-48-0
- Molecular formula:C14H8O4
- Molecular Weight:240.21
Yield:72-48-0 55%
Reaction Conditions:
Stage #1:phthalic anhydride;benzene-1,2-diol with aluminum (III) chloride;sodium chloride at 110 - 165; for 4 h;Friedel Crafts Acylation;
Stage #2: with hydrogenchloride in water at 0 - 100; for 0.75 h;Heating / reflux;
Steps:
A mixture of anhydrous AlCl3 (5 mmol, 0.667 g) and pre-baked NaCl (2.5 mmol, 0.145 g) was heated (110° C.) in an oil bath till molten. A homogenous mixture of phthalic anhydrides, (1 mmol) and substituted phenols, (1 mmol) were reacted with AlCl3/NaCl melt. The temperature was slowly increased and maintained at 165° C. for 4 h. The reaction mixture was cooled to 0° C., 10 ml of 10% HCl added, stirred for 15 min at 0° C. and refluxed at 100° C. for 30 min. The reaction mixture was cooled to room temperature and extracted with ethyl acetate. The resulting product was purified by C18 MPLC column using acetonitrile:water (1:1) as the mobile phase.; UV λmax (CH3OH) (log ε): 205 (4.47), 246 (4.6), 275 (4.4), 431 (3.7). 1H NMR (DMSO): δ 7.27 (d, 1H, J=8.5), 7.70 (d, 1H, J=8.5), 7.95 (m, 2H), 8.23 (m, 2H). 13C NMR (DMSO): 190.25, 182.03, 154.2, 152.28, 136.57, 135.51, 135.04, 134.16, 128.59, 127.95, 125.26, 122.70, 122.31, 117.73. Yield: 55%.
References:
Board of Trustees of Michigan State University US2005/267307, 2005, A1 Location in patent:Page/Page column 4, 5
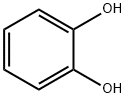
120-80-9
524 suppliers
$13.00/25g
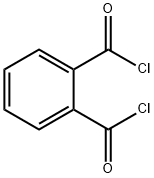
88-95-9
184 suppliers
$10.00/25 g

72-48-0
419 suppliers
$16.80/25G
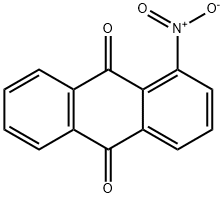
82-34-8
177 suppliers
$16.00/25g
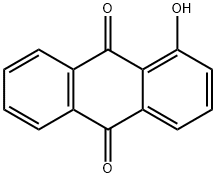
129-43-1
143 suppliers
$49.00/5g
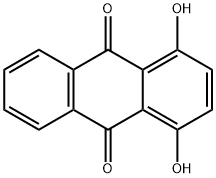
81-64-1
467 suppliers
$5.00/25g

72-48-0
419 suppliers
$16.80/25G
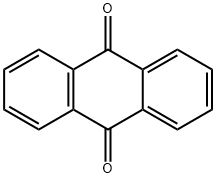
84-65-1
575 suppliers
$5.00/100mg

72-48-0
419 suppliers
$16.80/25G