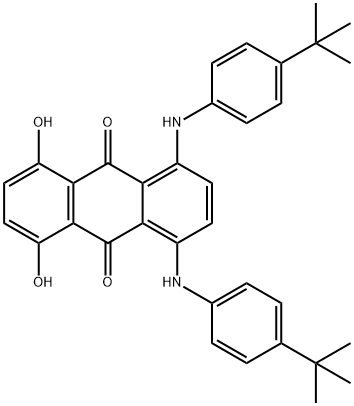
1,4-bis[[4-(1,1-dimethylethyl)phenyl]amino]-5,8-dihydroxyanthraquinone synthesis
- Product Name:1,4-bis[[4-(1,1-dimethylethyl)phenyl]amino]-5,8-dihydroxyanthraquinone
- CAS Number:4851-50-7
- Molecular formula:C34H34N2O4
- Molecular Weight:534.64
Yield:4851-50-7 23.1 g
Reaction Conditions:
with 1,3,4-trimethylimidazolidin-2-one;dipotassium hydrogenphosphate at 150 - 180; for 13 h;Reagent/catalyst;
Steps:
9 Example 1
General procedure: Example 1
Preparation of 1,4-bis(4-tert-butylphenylamino)-5,8-dihydroxyanthraquinone (in Analogy to EP-A 1 074 586, Non-Inventive)
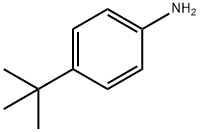
100 g (1010 mmol) of N-methylpyrrolidone were initially charged in a glass reactor under passing nitrogen and heated to 70° C. 20 g (134 mmol) of p-tert-butylaniline were then added.
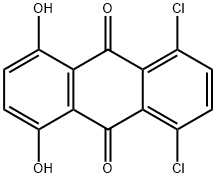
Subsequently, 17 g (44 mmol) of 5,8-dichloro-1,4-dihydroxyanthraquinone (technical-grade, 80%) and 15.7 g (110.5 mmol) of disodium hydrogenphosphate were added and the reaction mixture was heated to 150° C.
The reaction mixture was stirred at this temperature for 3 hours and then heated to 180° C. and stirred at this temperature for a further 10 hours.
After completion of the reaction, the reaction mixture was cooled to 100° C. and 30 g of methanol were added.
This mixture was stirred at 80° C. for one hour.
After cooling to 50° C., the mixture was filtered through a Nutsche filter.
The filter cake was firstly washed with 450 g of warm methanol and then with 1500 ml of warm water.
The isolated and washed product was then dried in a vacuum drying cabinet at 70° C. and 150 hPa.
Yield 22 g (93.5% of theory)
References:
US2019/161617,2019,A1 Location in patent:Paragraph 0052-0057