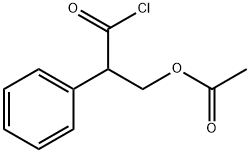
Acetyltropylic chloride synthesis
- Product Name:Acetyltropylic chloride
- CAS Number:14510-37-3
- Molecular formula:C11H11ClO3
- Molecular Weight:226.66
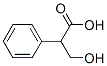
529-64-6
279 suppliers
$70.39/25 g
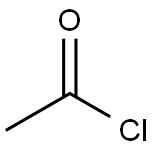
75-36-5
587 suppliers
$17.92/100G

14510-37-3
58 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:Tropic acid;acetyl chloride with N,N-dimethyl-formamide in dichloromethane at 25; for 3.5 h;Large scale;
Stage #2: with oxalyl dichloride in dichloromethane at 25; for 3 h;Large scale;
Steps:
Synthesis cmde atropine
Synthesis cmde atropineIn a reactor 2.0 kg of tropic acid was added to 6 L of dichloromethane and asuspension was formed. The suspension was stirred at 40 Hz at 25 °C. To the suspension0.1 Eq. of DMF was added. Acetyl chloride was slowly added over the course of at least30 minutes. The medium was stirred at 25 °C for 3 hours. 1.2 Eq. of oxalyl chloride(1.84kg) was then added to the medium over the course of at least 1 hour. The mediumwas stirred for 2 hours at 25 °C.The medium, containing acetyltropoyl chloride solution, was transferred to a clean container and stored at room temperature. In a reactor 2.0 kg of tropine was added to 6 L of dichloromethane to form asolution. The solution was stirred until it reached 35 °C. 1.0 Eq. of methanesulfonic acid (1.39kg) was added over the course of at least 30 minutes. The medium was stirred for 10mm at 35 °C to form a tropine methanesulfonate solution.The acetyltropoyl chloride solution was then transferred to the tropinemethanesulfonate solution and the resulting solution was stirred at reflux for at least 18 hours. The reaction medium was then cooled down to 35 °C. 12 L of a 1M solution of hydrochloric acid was added producing a biphasic mixture. The biphasic mixture wasstirred for at least 24 hours at 35 °C resulting in an aqueous layer containing atropine.The medium was then cooled down to 20 °C. The agitation was stopped for a 30 minute decantation and the organic layer was discarded. 2 L of new dichloromethane was added and the medium was stirred for 10 minutes. A second 30 minute decantation wasperformed and again the organic layer was discarded.The aqueous layer was cooled down to 5 °C and a 4M aqueous solution of sodium hydroxide was added over the course of at least 1 hour until the pH of the solution reached at least 13. A solid precipitate formed while the medium was stirred at 5 °C. The solidwas collected by filtration after 2 hours of stirring and then washed with 4 L of distilled water resulting in solid atropine (Formula I)(referred to as cmde atropine).In a reactor the cmde atropine was added to 14 L of distilled water to form asuspension. The suspension was stirred at 20 °C for at least 1 hour. The solid atropine was collected by filtration and washed with 5 L of distilled water.The crude atropine was dried under vacuum at 60 °C until water content was below 1.0% as measured by Karl Fischer titration. The yield was about 75%.
References:
ROUVER INVESTMENT S.à.R.L;COSTA, Franck Lopes;LHERMITTE, Hervé WO2016/16692, 2016, A1 Location in patent:Page/Page column 8

529-64-6
279 suppliers
$70.39/25 g

14510-37-3
58 suppliers
inquiry

529-64-6
279 suppliers
$70.39/25 g
108-24-7
0 suppliers
$14.00/250ML

14510-37-3
58 suppliers
inquiry