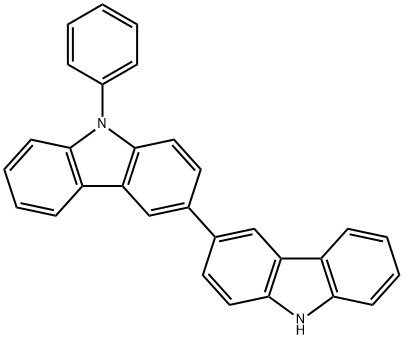
9-Phenyl-9H,9'H-[3,3']bicarbazolyl synthesis
- Product Name:9-Phenyl-9H,9'H-[3,3']bicarbazolyl
- CAS Number:1060735-14-9
- Molecular formula:C30H20N2
- Molecular Weight:408.49
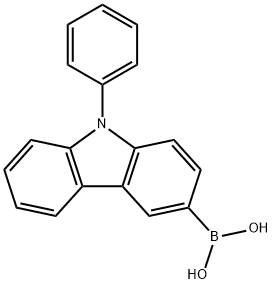
854952-58-2
290 suppliers
$18.50/250mg
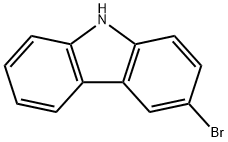
1592-95-6
404 suppliers
$5.00/1g
![9-Phenyl-9H,9'H-[3,3']bicarbazolyl](/CAS/GIF/1060735-14-9.gif)
1060735-14-9
181 suppliers
$27.00/250mg
Yield:1060735-14-9 97%
Reaction Conditions:
Stage #1:N-phenyl-9H-carbazol-3-boronic acid;3-bromo-9H-carbazole with potassium carbonate in ethanol;water;toluene for 0.5 h;Inert atmosphere;
Stage #2: with tris-(dibenzylideneacetone)dipalladium(0);triphenylphosphine in ethanol;water;toluene for 2 h;Reflux;
Steps:
1.1 Example 1 9,9'-Diphenyl-3,3'-bicarbazole
(1)3-Boronic acid-9-phenylcarbazole12.92 g (0.053 mol) of 3-bromocarbazole, 17.28 g (0.125 mol) of potassium carbonate and 100 mL of a solvent (toluene: EtOH: H 2 O = 10: 2: 1) The mixture was purged with nitrogen for 30 min, 0.046 g of Pd2 (dba) 3 and 0.026 g of triphenylphosphine were added and the mixture was heated to reflux. After 2 h, no starting material was detected by TLC. The reaction was terminated by adding water to the aqueous layer and the organic layer was filtered through silica gel to remove the catalyst Toluene was distilled off under reduced pressure, cooled to 13 ° C, suction filtered and dried in vacuo to give 19.80 g of 9-phenyl-3,3'-bicarbazole as a solid in 97% yield.
References:
Puyang Huicheng Electronic Materials Co., Ltd.;Feng Haidong;Wang Yongqiao;Wu Beihong;Li Zheng;Zhang Feifei CN106631984, 2017, A Location in patent:Paragraph 0020; 0021

1592-95-6
404 suppliers
$5.00/1g
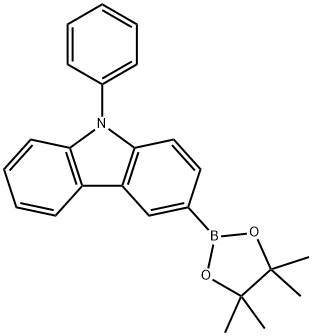
1126522-69-7
131 suppliers
$10.00/1g
![9-Phenyl-9H,9'H-[3,3']bicarbazolyl](/CAS/GIF/1060735-14-9.gif)
1060735-14-9
181 suppliers
$27.00/250mg
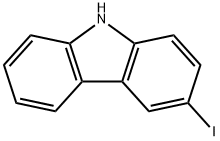
16807-13-9
175 suppliers
$9.00/1g

1126522-69-7
131 suppliers
$10.00/1g
![9-Phenyl-9H,9'H-[3,3']bicarbazolyl](/CAS/GIF/1060735-14-9.gif)
1060735-14-9
181 suppliers
$27.00/250mg

854952-58-2
290 suppliers
$18.50/250mg

16807-13-9
175 suppliers
$9.00/1g
![9-Phenyl-9H,9'H-[3,3']bicarbazolyl](/CAS/GIF/1060735-14-9.gif)
1060735-14-9
181 suppliers
$27.00/250mg
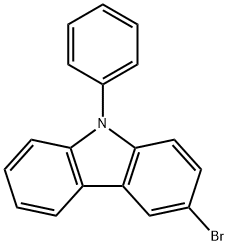
1153-85-1
322 suppliers
$7.00/1g
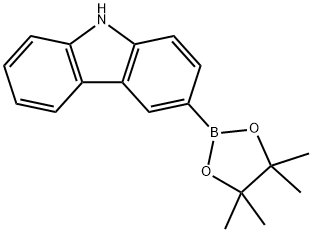
855738-89-5
141 suppliers
$11.00/1g
![9-Phenyl-9H,9'H-[3,3']bicarbazolyl](/CAS/GIF/1060735-14-9.gif)
1060735-14-9
181 suppliers
$27.00/250mg