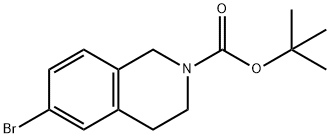
6-BROMO-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:6-BROMO-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:893566-74-0
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2
34784-05-9
323 suppliers
$5.00/1g

24424-99-5
863 suppliers
$13.50/25G

893566-74-0
110 suppliers
$28.00/250mg
Yield: 70.14%
Reaction Conditions:
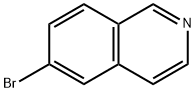
Stage #1:6-bromo-isoquinoline with lithium triethylborohydride in tetrahydrofuran at 0 - 25; for 2 h;Inert atmosphere;
Stage #2:di-tert-butyl dicarbonate with sodium carbonate in tetrahydrofuran;water; pH=9 - 10 at 0 - 25; for 15 h;
Steps:
4.1 Step 1: Synthesis of Compound WX135-2
Under a nitrogen atmosphere, at 0° C., to a solution of WX135-1 (2.00 g, 9.61 mmol) in tetrahydrofuran (20.00 mL) was added lithium triethylborohydride (1 M, 42.28 mL) dropwise, and the system was reacted at 25° C. for 2 hours. After the reaction was complete, the system was firstly adjusted to pH=2-3 with 1M diluted hydrochloric acid, and then adjusted to pH=9-10 with sodium carbonate solution. At 0° C., to the system was added Boc2O (4.19 g, 19.22 mmol, 4.42 mL), and the reaction mixture was stirred at 25° C. for 15 hours. After the reaction was complete, at 25° C., to the reaction system was added 100 mL water. The system was diluted with 100 mL ethyl acetate, and extracted with ethyl acetate (100 mL×3). The organic layers were combined, washed with brine (150 mL×2), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography (petroleum ether:ethyl acetate=100:1-50:1) to afford Compound WX135-2 (2.42 g, 70.14% yield), colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.33-7.29 (m, 2H), 6.99 (br d, J=8.0 Hz, 1H), 4.52 (s, 2H), 3.63 (br s, 2H), 2.81 (br t, J=5.6 Hz, 2H), 1.50 (s, 9H).
References:
Medshine Discovery Inc.;He, Haiying;Jiang, Zhigan;Shi, Weihua;Xia, Jianhua;Li, Jian;Chen, Shuhui US2019/375744, 2019, A1 Location in patent:Paragraph 0132; 0133
226942-29-6
152 suppliers
$19.00/250mg

24424-99-5
863 suppliers
$13.50/25G

893566-74-0
110 suppliers
$28.00/250mg
215798-19-9
111 suppliers
$16.00/100mg

24424-99-5
863 suppliers
$13.50/25G

893566-74-0
110 suppliers
$28.00/250mg
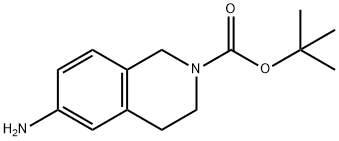
164148-92-9
146 suppliers
$11.00/100mg

893566-74-0
110 suppliers
$28.00/250mg

226942-29-6
152 suppliers
$19.00/250mg

24424-99-5
863 suppliers
$13.50/25G
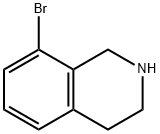
75416-51-2
93 suppliers
$24.00/100mg
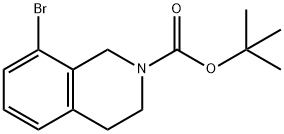
893566-75-1
71 suppliers
$11.00/100mg

893566-74-0
110 suppliers
$28.00/250mg