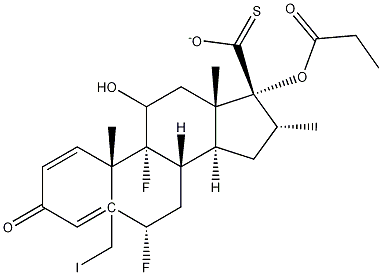
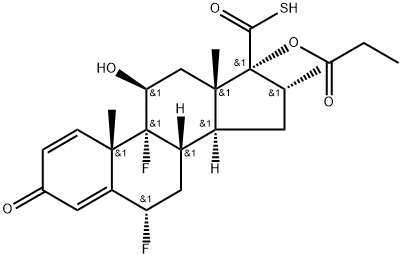
5-Iodomethyl 6a,9a-Difluoro-11-hydroxy-16a-methyl-3-oxo-17a-(propionyloxy)-androsta-1,4-diene-17-carbothioate synthesis
- Product Name:5-Iodomethyl 6a,9a-Difluoro-11-hydroxy-16a-methyl-3-oxo-17a-(propionyloxy)-androsta-1,4-diene-17-carbothioate
- CAS Number:80474-67-5
- Molecular formula:C25H31F2IO5S
- Molecular Weight:608.4769
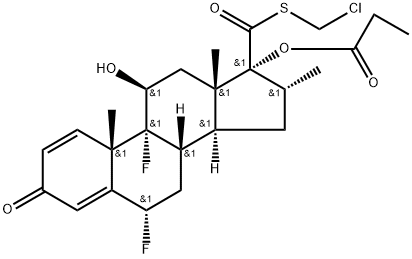
80486-69-7
33 suppliers
$210.00/5mg

80474-67-5
28 suppliers
$345.00/25mg
Yield:80474-67-5 75%
Reaction Conditions:
with 2,6-di-tert-butyl-4-methyl-phenol;hydroquinone;sodium iodide in acetone at 60 - 65; for 24 - 48 h;Product distribution / selectivity;
Steps:
1; 2; 3; 4; 5; 6
Examples; Example 1 : preparation ofiodomethyl ester 6 from chloromethyl ester 5; Sodium iodide (4.0 eq) was charged to acetone (20 vol) under stirring. Butylated hydroxytoluene (BHT) (1.0 eq) and hydroquinone (1.0 eq) were added to the stirred suspension of sodium iodide at 25-300C. The reaction mixture was stirred for 30 minutes. Chloromethyl ester 5 (1.0 eq) was added to this stirred suspension and the reaction mixture was refluxed for 24 hours at 60-650C. After completion of the reaction, the product was isolated by distillation of acetone and precipitation by adding 5% w/v solution of
References:
WO2007/144668,2007,A2 Location in patent:Page/Page column 21; 29-30
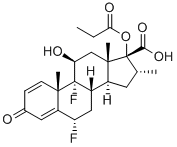
65429-42-7
106 suppliers
$110.00/10mg

80474-67-5
28 suppliers
$345.00/25mg
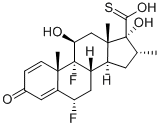
80473-92-3
91 suppliers
$198.00/50mg

80474-67-5
28 suppliers
$345.00/25mg
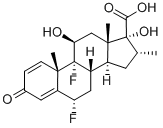
28416-82-2
227 suppliers
$60.00/50mg

80474-67-5
28 suppliers
$345.00/25mg
80474-45-9
111 suppliers
$501.81/5MG

80474-67-5
28 suppliers
$345.00/25mg