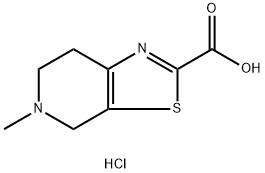
5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride synthesis
- Product Name:5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride
- CAS Number:720720-96-7
- Molecular formula:C8H11ClN2O2S
- Molecular Weight:234.7
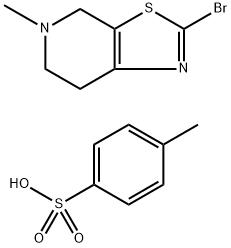
852291-42-0
18 suppliers
inquiry
1864-94-4
101 suppliers
$6.00/1g
![5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride](/CAS/20150408/GIF/720720-96-7.gif)
720720-96-7
332 suppliers
$6.00/250mg
Yield:720720-96-7 86.5%
Reaction Conditions:
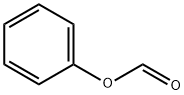
Stage #1:2-bromo-5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine p-toluenesulfonate;phenyl formate with palladium diacetate;triethylamine;4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene in acetonitrile at 60; for 39 h;Autoclave;Inert atmosphere;
Stage #2: with lithium hydroxide monohydrate in tetrahydrofuran at 20; for 2 h;
Stage #3: with hydrogenchloride in methanol at 20; for 2 h;Cooling with ice;
Steps:
8 Example 8
5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (1-c-hc1)
5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (1-c-hc1)
To a 100 mL autoclave, compound (1-br-ts) (5.0 g, 12.34 mmol), Pd(OAc)2 (2.8 mg, 0.0123 mmol), and xantphos (14.3 mg, 0.0247 mmol) were added.
In a glove box under a current of nitrogen, a solution containing phenyl formate (2.26 g, 18.50 mmol) and Et3N (5.1 mL, 36.99 mmol) in degassed Me3CN (20 mL:
the degassing was carried out by repeated reduction in pressure and purging with nitrogen three times) was added to the reaction mixture.
After sealing, the mixture was stirred at 60° C. for 39 hours.
After cooling of the reaction solution, toluene (50 mL) was added thereto, and the mixture was washed with 1% NaOH (50 mL) and 20% saline (25 mL) and concentrated into 10 mL under reduced pressure.
To the residue, THF (20 mL), H2O (2 mL), and LiOH.H2O (1.04 g, 2.0 equiv) were added, and the mixture was stirred at room temperature for 2 hours.
To the reaction solution, c-HCl (3.86 g, 3.5 eq.) and MeOH (50 mL) were added, and the slurry was stirred at room temperature for 1 hour and then under ice cooling for 1 hour and filtered.
The crystals obtained were washed with MeOH (10 mL) of 0 to 5° C. and dried under reduced pressure to obtain the title compound (2.51 g, 86.5%) as white crystals.
1H-NMR (500 Hz, DMSO-d6) δ: 4.63-4.55 (m, 2H), 3.62-3.57 (m, 2H), 3.23-3.14 (m, 2H), 2.94 (s, 3H).
References:
Daiichi Sankyo Company, Limited;UEDA, Tsuyoshi US2017/50983, 2017, A1 Location in patent:Paragraph 0197; 0198; 0199; 0200

852291-42-0
18 suppliers
inquiry
124-38-9
129 suppliers
$175.00/23402
![5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride](/CAS/20150408/GIF/720720-96-7.gif)
720720-96-7
332 suppliers
$6.00/250mg
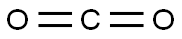
124-38-9
129 suppliers
$175.00/23402
![5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride](/CAS/20150408/GIF/720720-96-7.gif)
720720-96-7
332 suppliers
$6.00/250mg
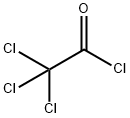
76-02-8
257 suppliers
$59.70/50g
![Thiazolo[5,4-c]pyridine, 4,5,6,7-tetrahydro-5-methyl-](/CAS/GIF/259809-24-0.gif)
259809-24-0
63 suppliers
$178.00/100mg
![5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride](/CAS/20150408/GIF/720720-96-7.gif)
720720-96-7
332 suppliers
$6.00/250mg

1445-73-4
461 suppliers
$15.00/25g
![5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride](/CAS/20150408/GIF/720720-96-7.gif)
720720-96-7
332 suppliers
$6.00/250mg