1-[2-NITRO-4-(TRIFLUOROMETHYL)PHENYL]HEXAMETHYLENIMINE synthesis
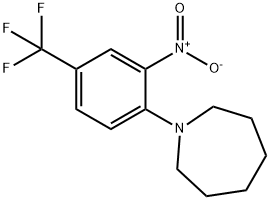
- Product Name:1-[2-NITRO-4-(TRIFLUOROMETHYL)PHENYL]HEXAMETHYLENIMINE
- CAS Number:62054-71-1
- Molecular formula:C13H15F3N2O2
- Molecular Weight:288.27
Yield:62054-71-1 97.5%
Reaction Conditions:
in N,N-dimethyl-formamide at 0 - 20; for 1 h;
Steps:
4-1
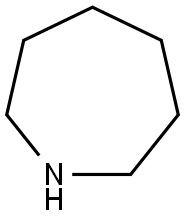
Hexahydro-1H-azepine (682 μl, 6.05 mmol, commercially available product) was added at 0°C to an N,N-dimethylformamide (DMF; 2 ml) solution of 1-fluoro-2-nitro-4-(trifluoromethyl)benzene (506 mg, 2.42 mmol, commercially available product). The resulting mixture was warmed to room temperature and stirred for one hour. Water was added to the mixture, and the resulting mixture was extracted three times with ethyl acetate. The obtained organic layer was washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (20 g, hexane/ethyl acetate = 7/1). Thus, hexahydro-1-[2-nitro-4-(trifluoromethyl)phenyl]-1H-azepine (680 mg, 97.5%) was yielded as an orange-colored solid. The results of TLC and 1H NMR (CDCl3, 400 MHz) are as follows: TLC Rf 0.49 (hexane/ethyl acetate = 5/1); 1H NMR (CDCl3, 400 MHz) δ 1.57-1.63 (m, 4H, 2CH2), 1.79-1.83 (m, 4H, 2CH2), 3.31 (t, 4H, J = 5.5 Hz, 2CH2), 7.11 (d, 1H, J = 9.1 Hz, aromatic) 7.53 (dd, 1H, J = 2.0, 9.1 Hz, aromatic), 7.99 (d, 1H, J = 2.0 Hz, aromatic).
References:
EP1712242,2006,A1 Location in patent:Page/Page column 33; 34