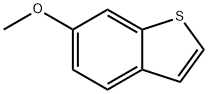
6-Methoxybenzo(b)thiophene synthesis
- Product Name:6-Methoxybenzo(b)thiophene
- CAS Number:90560-10-4
- Molecular formula:C9H8OS
- Molecular Weight:164.22
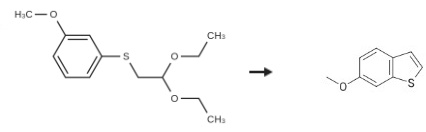
![Benzene, 1-[(2,2-diethoxyethyl)thio]-3-Methoxy-](/CAS/GIF/96803-85-9.gif)
96803-85-9
7 suppliers
inquiry

90560-10-4
97 suppliers
$45.00/50mg
Yield:90560-10-4 63%
Reaction Conditions:
Stage #1: 1-[(2,2-diethoxyethyl)sulfanyl]-3-methoxybenzenewith methanesulfonic acid;Celite in hexane; for 1 h;Heating / reflux;
Stage #2:
Stage #3: with triethylamine at 20;
Steps:
1a.B Example (1a): 6-methoxybenzo[b]thiophene; Method B
Method B: A solution of the crude 1- (2, 2-diethoxyethylsulfanyl)-3-methoxybenzene (8.27 g, 32.3 mmole) in hexane (100 ml) was added, dropwise, to a solution of [METHANESULFONIC] acid (1.05 [ML,] 1.55 g, 16.1 mmole) in hexane (1000 [ML)] containing 16.5 [G] of celite (2 wt. eq. ). The resultant solution was heated at reflux for one hour. After cooling to room temperature, the reaction was quenched by addition of Et3N (4.5 ml, 3.26 g, 32.3 [MMOLE).] The crude reaction mixture was filtered and the filtrate was concentrated, in vacuo, to give a red oil which was purified by silica gel chromatography. Elution with hexane: [ET20] (98: 2) and evaporation of the appropriate fractions gave 3.35 g (63%) of a [COLORLESS OIL.'H] NMR [(DMSO-D6)] 8 7.74 [(1 H,] d, [J=8. 7HZ),] 7.56 [(1 H,] d, [J = 2. 3 HZ),] 7.52 [(1 H,] d, [J=5. 3HZ),] 7.33 (1 H, d, J = 5.3Hz), 6.99 (1 H, dd, J = 2.3, 8.7 Hz), 3.81 (3H, s). Anal. Calcd. for [C9HBOS :] C, 65.82 ; H, 4.91 ; S, 19.53. Found: C, 66.01 ; H, 5.00 ; S, 19.40.
References:
WO2003/106462,2003,A1 Location in patent:Page 34
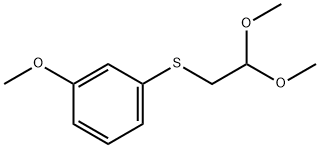
![Acetaldehyde, 2-[(3-methoxyphenyl)thio]-](/CAS/20210305/GIF/105126-91-8.gif)
105126-91-8
0 suppliers
inquiry

90560-10-4
97 suppliers
$45.00/50mg
![Benzene, 1-[(2,2-diethoxyethyl)thio]-3-Methoxy-](/CAS/GIF/96803-85-9.gif)
96803-85-9
7 suppliers
inquiry

90560-10-4
97 suppliers
$45.00/50mg
![4-Methoxybenzo[b]thiophene](/CAS/GIF/3781-90-6.gif)
3781-90-6
33 suppliers
$179.00/100mg
98733-08-5
5 suppliers
inquiry

90560-10-4
97 suppliers
$45.00/50mg
15570-12-4
240 suppliers
$17.00/1g
2032-35-1
482 suppliers
$5.00/10g

90560-10-4
97 suppliers
$45.00/50mg
