
6-Carboxyfluorescein synthesis
- Product Name:6-Carboxyfluorescein
- CAS Number:3301-79-9
- Molecular formula:C21H12O7
- Molecular Weight:376.32
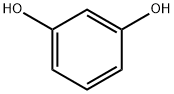
108-46-3
788 suppliers
$10.00/10g
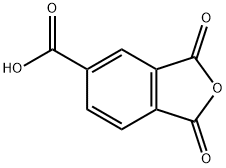
552-30-7
422 suppliers
$16.00/25g

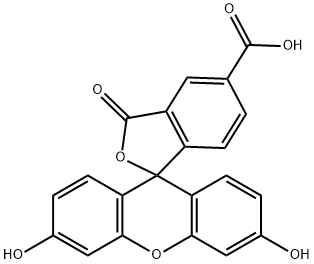
3301-79-9
242 suppliers
$49.00/100mg
76823-03-5
271 suppliers
$13.00/25mg
Yield:-
Reaction Conditions:
with sulfuric acid at 130; for 0.04 h;Irradiation;Title compound not separated from byproducts;
References:
El'tsov, A. V.;Martynova, V. P.;Sokolova, N. B.;Dmitrieva, N. M.;Brykov, A. S [Russian Journal of General Chemistry,1995,vol. 65,# 3.2,p. 454 - 456][Zhurnal Obshchei Khimii,1995,vol. 65,# 3,p. 511 - 513]
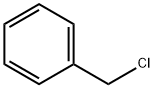
100-44-7
660 suppliers
$13.50/250G

108-46-3
788 suppliers
$10.00/10g

552-30-7
422 suppliers
$16.00/25g

3301-79-9
242 suppliers
$49.00/100mg

76823-03-5
271 suppliers
$13.00/25mg