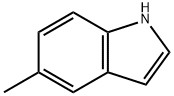
5-Methylindole synthesis
- Product Name:5-Methylindole
- CAS Number:614-96-0
- Molecular formula:C9H9N
- Molecular Weight:131.17
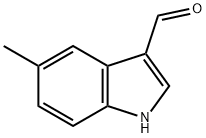
52562-50-2
140 suppliers
$15.00/250mg

614-96-0
320 suppliers
$19.00/1g
Yield:614-96-0 80%
Reaction Conditions:
with perchloric acid adsorbed on silica gel;anthranilic acid amide in acetonitrile at 80; for 6 h;
Steps:
Typical procedure for the deformylation of indole and azaindole-3-carboxaldehydes
General procedure: Solid acid catalyst (50% w/w) was added to the solution of indole-3-carboxaldehydes 1a-1h or azaindole-3-carboxaldehyde 6 (1 mmol) and anthranilamide 2a (1 mmol) in acetonitrile (6 mL). The reaction mixture was allowed to stir for 6-24 h at room temperature or at reflux temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, it was allowed to cool and catalyst was recovered by filtration. The filtrate was concentrated to get crude products which after silica gel (No.100-200) column chromatography gave deformylated products 4a-4h or 5 in 25-90% yield.
References:
Yadav, Rammohan R.;Battini, Narsaiah;Mudududdla, Ramesh;Bharate, Jaideep B.;Muparappu, Nagaraju;Bharate, Sandip B.;Vishwakarma, Ram A. [Tetrahedron Letters,2012,vol. 53,# 17,p. 2222 - 2225] Location in patent:experimental part
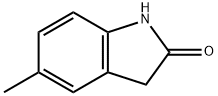
3484-35-3
139 suppliers
$19.00/100mg

614-96-0
320 suppliers
$19.00/1g
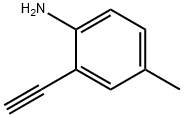
215589-37-0
38 suppliers
$43.00/100mg

614-96-0
320 suppliers
$19.00/1g
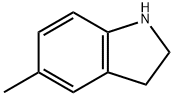
65826-95-1
100 suppliers
$75.00/100mg

614-96-0
320 suppliers
$19.00/1g
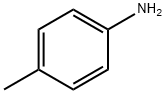
106-49-0
410 suppliers
$5.00/5 g

107-21-1
1368 suppliers
$10.00/25g
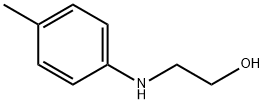
2933-74-6
11 suppliers
inquiry

614-96-0
320 suppliers
$19.00/1g
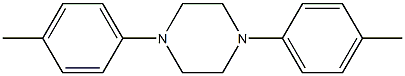
3367-48-4
0 suppliers
inquiry
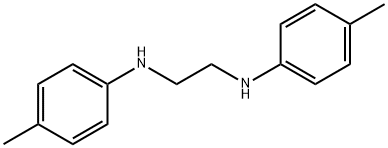
4693-68-9
3 suppliers
inquiry