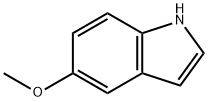
5-Methoxyindole synthesis
- Product Name:5-Methoxyindole
- CAS Number:1006-94-6
- Molecular formula:C9H9NO
- Molecular Weight:147.17
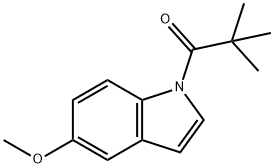
845619-77-4
2 suppliers
inquiry

1006-94-6
508 suppliers
$10.00/5g
Yield:1006-94-6 99%
Reaction Conditions:
with water;1,8-diazabicyclo[5.4.0]undec-7-ene in tetrahydrofuran; for 18 h;Reflux;
Steps:
4.3. General procedures for the deprotection of N-pivaloylindoles
General procedure: Method C: DBU (4 equiv) and water (2 equiv) was added to a solution of 1-pivaloyl derivatives (1 equiv) in THF (3 mL×mmol) and was stirred to reflux until the disappearance of the starting indole. The reaction mixture was eluyed with EtOAc (10 mL) and was washed with a saturated aqueous NH4Cl solution (4 mL×mmol). The aqueous layer was extracted with EtOAc (2×15 mL). The combined organic layers were dried over Na2SO4 and evaporated to afford the corresponding unprotected compound.
References:
Ruiz, Míriam;Sánchez, J. Domingo;López-Alvarado, Pilar;Menéndez, J. Carlos [Tetrahedron,2012,vol. 68,# 2,p. 705 - 710] Location in patent:experimental part
4382-54-1
243 suppliers
$10.00/250mg

1006-94-6
508 suppliers
$10.00/5g
![5-methoxy-1-[(4-methylphenyl)sulfonyl]-1H-Indole](/CAS/20180703/GIF/139717-71-8.gif)
139717-71-8
17 suppliers
inquiry

1006-94-6
508 suppliers
$10.00/5g
10601-19-1
356 suppliers
$10.00/5g

1006-94-6
508 suppliers
$10.00/5g
10242-01-0
167 suppliers
$12.00/250mg

1006-94-6
508 suppliers
$10.00/5g
