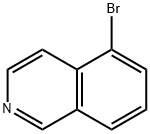
5-Bromoisoquinoline synthesis
- Product Name:5-Bromoisoquinoline
- CAS Number:34784-04-8
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
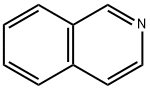
119-65-3
417 suppliers
$5.00/25g

34784-04-8
364 suppliers
$7.00/1g
Yield:34784-04-8 81%
Reaction Conditions:
Stage #1:isoquinoline with N-Bromosuccinimide;sulfuric acid at -25 - 20;
Stage #2: with ammonia in water; pH=8 - 10 at 0;
Steps:
4 Synthesis of 5-bromoisoquinoline
Synthesis of 5-bromoisoquinoline Into a 250 mL 3-necked round-bottom flask, was placed H2SO4 (150 mL). To the above was added isoquinoline (17 g, 131.62 mmol) in several batches, while cooling to a temperature of 0° C. To the above was added NBS (29.2 g, 164.04 mmol) in several batches, while cooling to a temperature of -25-22° C. The resulting solution was allowed to react, with stirring, for 2 h while the temperature was maintained at -25° to -22° C. The resulting solution was allowed to react with stirring overnight, while the temperature was maintained at room temperature. The reaction progress was monitored by TLC (EtOAc/PE=1:5). The reaction mixture was then quenched by the adding 1000 mL of H2O/ice. Adjustment of the pH to 8-10 was accomplished by the addition of NH3.H2O (30%). The resulting solution was extracted four times with 500 mL of EtOAc and the organic layers combined and dried over Na2SO4. The residue was purified by eluding through a column with a 1:5 EtOAc/PE solvent system. This resulted in 22.24 g (81%) of 5-bromoisoquinoline as a white solid.
References:
MEMORY PHARMACEUTICALS CORPORATION US2008/318941, 2008, A1 Location in patent:Page/Page column 22
1125-60-6
251 suppliers
$6.00/1g

34784-04-8
364 suppliers
$7.00/1g
![N-[(3-Bromophenyl)methylene]-2,2-dimethoxyethanamine](/CAS2/GIF/497863-61-3.gif)
497863-61-3
15 suppliers
$125.00/500 mg

34784-04-8
364 suppliers
$7.00/1g
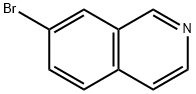
58794-09-5
180 suppliers
$39.00/500mg
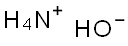
1336-21-6
730 suppliers
$14.56/250ML

1125-60-6
251 suppliers
$6.00/1g

34784-04-8
364 suppliers
$7.00/1g
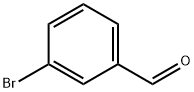
3132-99-8
502 suppliers
$5.00/10g
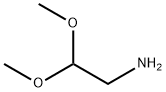
22483-09-6
498 suppliers
$5.00/5g

34784-04-8
364 suppliers
$7.00/1g

58794-09-5
180 suppliers
$39.00/500mg