
5-BROMOINDOLINE synthesis
- Product Name:5-BROMOINDOLINE
- CAS Number:22190-33-6
- Molecular formula:C8H8BrN
- Molecular Weight:198.06
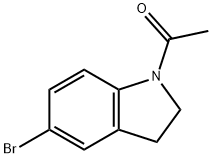
22190-38-1
132 suppliers
$40.10/1g

22190-33-6
247 suppliers
$9.00/1g
Yield:22190-33-6 98.04%
Reaction Conditions:
with hydrogenchloride in ethanol;water at 78; for 4 h;Green chemistry;Solvent;Temperature;
Steps:
1.4 (4) Synthesis of 5-bromoindoline
A) A mixture of 38 g (0.16 mol) of N-acetyl-5-bromoindoline, 31 g of concentrated hydrochloric acid and 41 g of ethanol was charged into a reaction flask and stirred uniformly to obtain the reaction mixture I; B) reaction mixture I at 78 , reaction 4h, the chromatographic monitoring of raw materials disappeared; C) neutralized with 55 g of sodium hydroxide solution; D) to obtain organic phase J and water phase K; E) The organic phase J was extracted three times with 445 g of chloroform. The organic layers were combined and the solvent was recovered to give 30.74 g of 5-bromoindoline, yield 98.04% and content ≥99%.
References:
Sinosteel Anshan Research Institute of Thermo-energy Co., Ltd.;Wang, Shoukai;Wang, Haiyang;Zhao, Sujuan;Zhao, Wei;Ma, Saiyong;Xu, Haoran;Jin, Dan;Chen, Xing CN106432040, 2017, A Location in patent:Paragraph 0108-0113; 0135-0140; 0162-0167
10075-50-0
689 suppliers
$5.00/10g

22190-33-6
247 suppliers
$9.00/1g
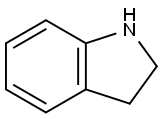
496-15-1
427 suppliers
$8.00/10g

22190-33-6
247 suppliers
$9.00/1g

22190-38-1
132 suppliers
$40.10/1g

22190-33-6
247 suppliers
$9.00/1g

64-17-5
727 suppliers
$10.00/50g
918529-91-6
2 suppliers
inquiry

22190-33-6
247 suppliers
$9.00/1g