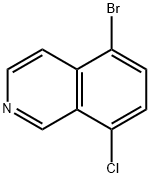
5-BROMO-8-CHLOROISOQUINOLINE synthesis
- Product Name:5-BROMO-8-CHLOROISOQUINOLINE
- CAS Number:956003-79-5
- Molecular formula:C9H5BrClN
- Molecular Weight:242.5
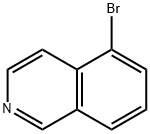
34784-04-8
364 suppliers
$7.00/1g

956003-79-5
53 suppliers
$152.00/100mg
Yield:956003-79-5 96 %
Reaction Conditions:
with N-chloro-succinimide;sulfuric acid at 0 - 80;
Steps:
27.2 Step 2. 5-bromo-8-chloroisoquinoline (27.2)
To a solution of 5-bromoisoquinoline (27.1) (20.5g, 0.0985mol, 1.0eq) in H2SO4 (5.0 ml) at 0°C was added N-Chlorosuccinamide (19.75g, 1.5eq). After stirring at 80°C for 2 h, the reaction mixture was poured into water (50 ml), neutralized with concentrated ammonium hydroxide, and extracted with ethyl acetate (100 ml x 3). The combined organic layer was dried over sodium sulphate and concentrated under reduced pressure to afford 27.2 (18.5, 96%) MS (ES): m/z 243.93 [M+H]+
References:
WO2022/174253,2022,A1 Location in patent:Paragraph 00539
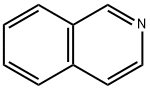
119-65-3
416 suppliers
$5.00/25g

956003-79-5
53 suppliers
$152.00/100mg