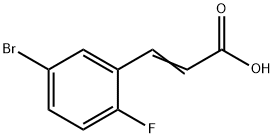
5-BROMO-2-FLUOROCINNAMIC ACID synthesis
- Product Name:5-BROMO-2-FLUOROCINNAMIC ACID
- CAS Number:202865-71-2
- Molecular formula:C9H6BrFO2
- Molecular Weight:245.05
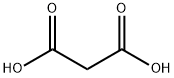
141-82-2
760 suppliers
$5.00/25g
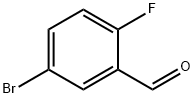
93777-26-5
362 suppliers
$5.00/5g

202865-71-2
104 suppliers
$6.00/1g
Yield: 1.3 g
Reaction Conditions:
with piperidine;pyridine at 100; for 3 h;
Steps:
8.1 Step 1: 3-(5-bromo-2-fluoro-phenyl)-acrylic acid
5-Bromo-2-fluoro-benzaldehyde (5.0 g, 24.6 mmol), malonic acid (5.6 g, 53.7 mmol), and piperidine (0.5 mL, 4.9 mmol) were added to pyridine (11.4 mL). The reaction mixture was stirred at 100° C. for 3 hours and then concentrated under reduced pressure. Distilled water was added to the reaction mixture, which was then filtered. The resulting solid was recrystallized from ethanol to give 1.3 g of the titled compound as a white solid. (0164) 1H NMR (400 MHz, CD3OD) 7.89 (d, 1H), 7.74-7.70 (m, 2H), 7.15 (t, 1H), 6.62 (d, 1H)
References:
YUHAN CORPORATION;Park, Chan-Sun;Kim, Young-Hwan;Lee, Gyu-Jin;Hur, Youn;Jung, Eun-Hye;Tak, Hee-Jae;Shin, Seung-Yub;Lee, Ho-Jin;Lee, Chun-Ho;Lee, Koo-Yeon US2015/291563, 2015, A1 Location in patent:Paragraph 0163; 0164