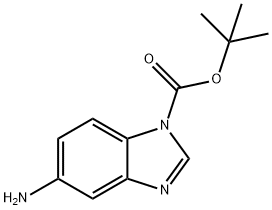
5-AMINO-1-BOC-BENZOIMIDAZOLE synthesis
- Product Name:5-AMINO-1-BOC-BENZOIMIDAZOLE
- CAS Number:297756-31-1
- Molecular formula:C12H15N3O2
- Molecular Weight:233.27

24424-99-5
861 suppliers
$13.50/25G
934-22-5
171 suppliers
$21.00/1g

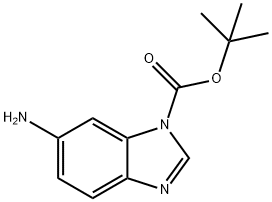
297756-31-1
23 suppliers
$208.00/250mg
297756-32-2
11 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 5-aminobenzimidazolewith sodium hydride in DMF (N,N-dimethyl-formamide) at 20; for 1 h;
Stage #2: di-tert-butyl dicarbonate in DMF (N,N-dimethyl-formamide) at 0 - 20; for 18 h;
Steps:
3 EXAMPLE 3; 8-[(Z and E)-4-Toluidinomethylidene]-6,8-dihydroimidazo[4,5-e]indol-7(3H)-one
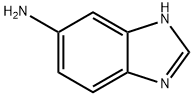
Sodium hydride (7.2 g, 60% in mineral oil, 0.18 mol) was added in portions over 30 min to a stirred solution of 5-aminobenzimidazole (15.0 g, 0.105 mol) in dry DMF (100 mL) and the mixture was stirred at room temperature for 30 min. The reaction mixture was cooled to 0° C. and a solution of di-tert-butyl dicarbonate (24.0 g, 0.110 mol) in dry DMF (25 mL) was added over 10 min. The reaction mixture was stirred at room temperature for 18 h. The solvent was evaporated under reduced pressure and the residue was partitioned between water (200 mL) and diethyl ether (200 mL). The organic phase was separated and the aqueous layer was extracted with diethyl ether. The combined organic extracts were washed with water and brine and dried over anhydrous magnesium sulfate. The drying agent was removed by filtration through a pad of silica gel and the filtrate evaporated to give a mixture of 5- and 6-amino-1-tert-butoxycarbonylbenzimidazole, 21.5 g (84%). H1 NMR (DMSO-d6): δ 8.40 (s, 1H), 8.23 (s, 1H), 7.55 (d, 1H, J=8.8 Hz), 7.34 (d, 1H, J=8.4 Hz), 7.13 (d, 1H, J=1.6 Hz), 6.83 (d, 1H, J=2 Hz), 6.68 (dd, 1H, J=8.8, 1.6 Hz), 6.60 (dd, 1H, J=8.4, 2 Hz), 1.61 (s, 9H), 1.60 (s, 9H).
References:
US6350747,2002,B1 Location in patent:Page column 50-51
![tert-butyl 5-nitro-1H-benzo[d]imidazole-
1-carboxylate](/CAS/20180527/GIF/1425932-17-7.gif)
1425932-17-7
1 suppliers
inquiry
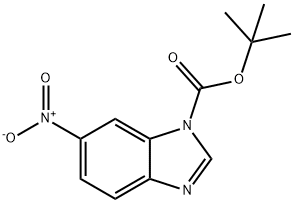
1442459-46-2
0 suppliers
inquiry

297756-31-1
23 suppliers
$208.00/250mg

297756-32-2
11 suppliers
inquiry