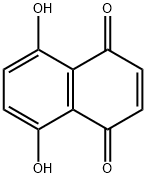
5,8-Dihydroxy-1,4-naphthoquinone synthesis
- Product Name:5,8-Dihydroxy-1,4-naphthoquinone
- CAS Number:475-38-7
- Molecular formula:C10H6O4
- Molecular Weight:190.15
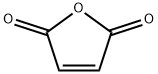
108-31-6
871 suppliers
$13.47/50 G
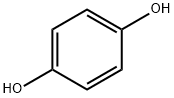
123-31-9
849 suppliers
$13.00/25g

475-38-7
144 suppliers
$65.00/250 mg
Yield: 16.95%
Reaction Conditions:
with aluminum (III) chloride;sodium chloride at 200; for 2 h;High pressure;Friedel-Crafts Acylation;Temperature;
Steps:
1 Example 1 The method for the synthesis of naphthoquinone by molten salt method is as follows:
In the first step, the oven will be preheated to 160°C.Weigh the sample with 25% fullness of the hydrothermal reactor, including NaCl 2.10g, with a balance.Anhydrous AlCl 310.67g, maleic anhydride 1.18g and hydroquinone 1.10g;The weighed sample is placed in a grinding crucible, fully mixed, ground, and transferred to a hydrothermal reactor.The reaction vessel was screwed; the reaction vessel was placed in an oven that had been preheated to 200 DEG C. and subjected to Friedel-Crafts acylation reaction for 2 hours.After the reaction is complete, remove the reactor and allow the reactor vessel to cool to room temperature.A purple-black solid was obtained as a complex of 5,8-dihydroxy-1,4-naphthoquinone and tri-alumina;In the second step, transfer the purple-red solid obtained in the first step to a beaker.Add 20% hydrochloric acid solution to fully boil until bubbles are no longer generated, then filter to remove the upper solid;The solid was then dispersed with a small amount of ultrapure water and extracted three times with CH2Cl2.The resulting extract was dried under reduced pressure and dried in a vacuum oven under vacuum at 55°C for about 8 hours.0.3223 g of purple-red powder was obtained, namely naphthoquinone (5,8-dihydroxy-1,4-naphthoquinone).The theoretical yield of hydroquinone was 1.8998 g as the starting material, so the total yield was 16.95%.
References:
Yunnan China National Tobacco Industry Co., Ltd.;Hubei University;Chang Gang;Zhou Yang;Zhu Ruizhi;Liu Zhihua;Cao Yunbin;Cao Ribing CN107417508, 2017, A Location in patent:Paragraph 0028-0031; 0033-0042
10075-68-0
6 suppliers
inquiry

475-38-7
144 suppliers
$65.00/250 mg
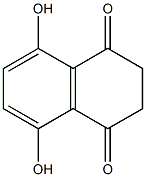
4988-51-6
0 suppliers
inquiry

475-38-7
144 suppliers
$65.00/250 mg
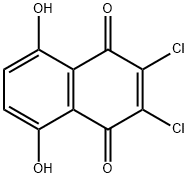
14918-69-5
76 suppliers
$31.00/100mg

475-38-7
144 suppliers
$65.00/250 mg
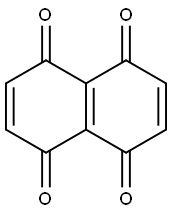
23077-93-2
2 suppliers
inquiry

475-38-7
144 suppliers
$65.00/250 mg