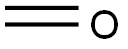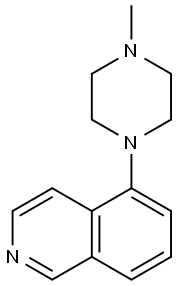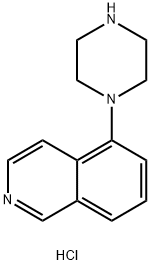
5-(1-piperazinyl)-isoquinoline HCl synthesis
- Product Name:5-(1-piperazinyl)-isoquinoline HCl
- CAS Number:209733-17-5
- Molecular formula:C13H16ClN3
- Molecular Weight:249.74

24424-99-5
863 suppliers
$13.50/25G

209733-17-5
22 suppliers
inquiry
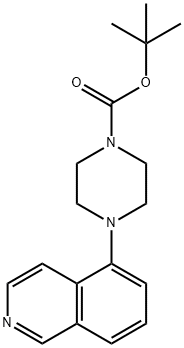
444620-69-3
23 suppliers
inquiry
Yield:444620-69-3 0.86 g
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;Cooling with ice;
Steps:
To 5-(piperazin-l-yl)isoquinoline, HCl (0.58 g, 2.322 mmol) and NaOH (5.1 1 mL, 5.11 mmol) in dioxane (6 mL), cooled in ice bath, was added BOC2O (0.539 mL, 2.322 mmol) in dioxane (6 mL). The organics were stripped and the reaction was partitioned with H2O (30mL) and EtOAc (100 mL). The organic layer was washed with brine (15 mL) and dried (MgSO4). Collected Boc-protected compound as a yellow oil (0.86 g) which was then hydrogenated at 55 psi with PtO2 in EtOH. The crude product was then filtered through Celite and collected 0.73g (99%) of the desired product as a off-white solid. MS (ESI) m/z: 318.1 (M+H)+.
References:
WO2013/56060,2013,A1 Location in patent:Paragraph 00269

24424-99-5
863 suppliers
$13.50/25G

209733-17-5
22 suppliers
inquiry
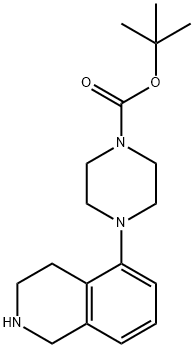
444620-71-7
1 suppliers
inquiry