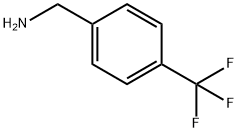
4-(Trifluoromethyl)benzylamine synthesis
- Product Name:4-(Trifluoromethyl)benzylamine
- CAS Number:3300-51-4
- Molecular formula:C8H8F3N
- Molecular Weight:175.15
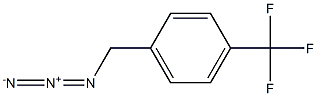
222716-19-0
1 suppliers
inquiry

3300-51-4
229 suppliers
$10.00/5G
Yield:3300-51-4 93%
Reaction Conditions:
with 1-phenylphospholane-1-oxide;1,3-diphenyl-disiloxane in tetrahydrofuran at 23; for 24 h;Staudinger Azide Reduction;Reagent/catalyst;Solvent;
Steps:
Additive-Free Catalytic Staudinger Reduction at Ambient Temperature; General Procedure
General procedure: To a 2-dram vial equipped with a stir bar were added all solid reagents(azide: 0.25 mmol, 1.0 equiv), 1a (4.5 mg, 0.025 mmol, 0.1equiv), THF (1 mL), all liquid reagents (azide: 0.25 mmol, 1.0 equiv),and then DPDS (57.5 mg, 0.25 mmol, 1.0 equiv). The reaction flaskwas sealed with a PTFE septum in the screwcap and the mixture wasstirred at 23 °C for 24 h. The mixture was diluted with acetone andthe vessel was washed with acetone (7.5 mL); this mixture was loadeddirectly onto silica gel (200 mg). Purification by flash column chromatography(EtOAc/hexanes, 40:60 with 1.5-5% Et3N) afforded amine products 4.
References:
Buonomo, Joseph A.;Cole, Malcolm S.;Eiden, Carter G.;Aldrich, Courtney C. [Synthesis,2020,vol. 52,# 23,p. 3583 - 3594]
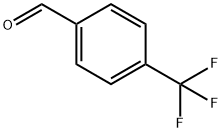
455-19-6
421 suppliers
$6.00/5g

3300-51-4
229 suppliers
$10.00/5G
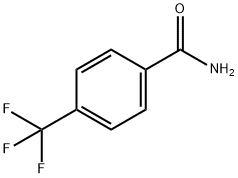
1891-90-3
143 suppliers
$9.00/5g

3300-51-4
229 suppliers
$10.00/5G
349-95-1
267 suppliers
$14.00/5g

3300-51-4
229 suppliers
$10.00/5G
455-18-5
360 suppliers
$5.00/5g

3300-51-4
229 suppliers
$10.00/5G