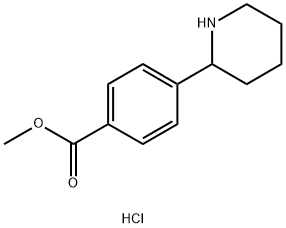
4-PIPERIDIN-2-YL-BENZOIC ACID METHYL ESTER HCL synthesis
- Product Name:4-PIPERIDIN-2-YL-BENZOIC ACID METHYL ESTER HCL
- CAS Number:863769-42-0
- Molecular formula:C13H18ClNO2
- Molecular Weight:255.74

863769-42-0
16 suppliers
inquiry

24424-99-5
841 suppliers
$13.50/25G
![1-Piperidinecarboxylic acid, 2-[4-(methoxycarbonyl)phenyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/863769-43-1.gif)
863769-43-1
1 suppliers
inquiry
Yield:863769-43-1 96%
Reaction Conditions:
with triethylamine in acetonitrile at 20; for 1 h;
Steps:
3
To a solution of 4-piperidin-2-yl-benzoic acid methyl ester HCl (116 mg) and triethylamine (192 al, 3 eq) in acetonitrile was added (BOC) 20 (120 mg, 1.2 eq). The reaction mixture was stirred at RT for 1 hour. The solvent was evaporated. The residue was purified by flash chromatography (DCM/MeOH 99/1 to 98/2), yielding the N-BOC- 4-piperidin-2-yl-benzoic acid methyl ester as a white powder (96% yield) The N-BOC-4-piperidin-2-yl-benzoic acid methyl ester (140 mg) was dissolved in a mixture of MeOH (5 ml) and aqueous 1M NaOH (3 ml). The reaction mixture was heated at 60°C for 1 hour, then cooled down at RT. MeOH was evaporated. The solution was acidified with 2M HCI. The product was extracted with DCM. The combined organic layers was evaporated. The N-BOC-4-piperidin-2-yl-benzoic acid was used for the next step without further purification. The N-BOC-4-piperidin-2-yl-N-pyridin-4-yl-benzamide was obtained in a similar manner as described in Example 29, using N-BOC-4-piperidin-2-yl-benzoic acid and 4- aminopyridine, yielding a pale yellow powder (80% yield). The N-BOC-4-piperidin-2-yl-N-pyridin-4-yl-benzamide was dissolved in aqueous 3M HCl (5 ml). The reaction mixture was heated at 55°C for 2 hours. The reaction mixture was cooled down at RT, and then washed with DCM (3 ml). The aqueous layer was evaporated under reduced pressure, yielding the title product as a white powder (98% yield). 1H NMR (300 MHz, D20) : 1.60-2. 08 ppm (m, 6H); 3.10 ppm (m, 1H) ; 3.45 ppm (m, 1H); 7.53 ppm (d, 2H, J = 8.4Hz) ; 7.90 ppm (d, 2H, J = 8.4Hz) ; 8.16 ppm (d, 2H, J = 7. 5Hz) ; 8.51 ppm (d, 2H, J = 7. 5Hz).
References:
WO2005/82367,2005,A1 Location in patent:Page/Page column 61

863769-42-0
16 suppliers
inquiry
![[4-(piperidin-2-yl)phenyl]methanol](/CAS/20200401/GIF/421552-43-4.gif)
421552-43-4
1 suppliers
inquiry