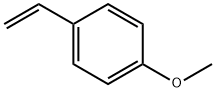
4-Methoxystyrene synthesis
- Product Name:4-Methoxystyrene
- CAS Number:637-69-4
- Molecular formula:C9H10O
- Molecular Weight:134.18
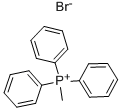
P-methoxy styrene is prepared by taking p-methoxy benzyl alcohol as a raw material and reacting with triphenylphosphine, formaldehyde and hydrobromic acid through the Wittig reaction.
P-methoxyacetophenone is used as a raw material, reduced to 1- (4-methoxyphenyl) ethanol by potassium borohydride, esterified with excessive potassium bisulfate in cyclohexane to generate sulfonic acid-1- (4-methoxyphenyl) ethyl ester, and finally subjected to elimination reaction to obtain 4-Methoxystyrene.
In 2021, an invention discloses a method for synthesizing 4-methoxystyrene, which comprises the steps of using p-methoxyacetophenone as a raw material, firstly carrying out hydrogenation reduction to generate 1- (4-methoxyphenyl) ethanol, then carrying out dehydration elimination reaction to obtain a crude product, and finally rectifying to obtain the 4-methoxystyrene. The invention adopts a catalytic hydrogenation method to get the intermediate 1- (4-methoxyphenyl) ethanol. The reaction selectivity of the step is excellent and can reach 98-99%, and meanwhile, hydrogen is used as a cleaning gas so that environmental pollution is avoided and industrial production is facilitated.
Yield:637-69-4 91%
Reaction Conditions:
Stage #1:Methyltriphenylphosphonium bromide with n-butyllithium in tetrahydrofuran at -78; for 0.25 h;Inert atmosphere;
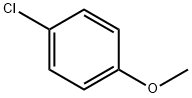
Stage #2:4-methoxy-benzaldehyde in tetrahydrofuranInert atmosphere;
Steps:
5.1.2. General procedure for styrene preparation
General procedure: To a stirred suspension of methyl triphenylphosphonium bromide(1.5 equiv.) in THF at -78 °C under argon atmosphere, n-butyllithium(1.5 equiv. 1.6 M in THF) was added drop wise. The yellow solution wasallowed to stir for 15 min before addition of the aldehyde (1 eq.) inTHF. upon its addition, the mixture turned white or pale yellow. After2 h (or observed completion of the reaction by TLC), saturated ammoniumchloride was added and the mixture was extracted withCH2Cl2. The combined organic extracts were then dried (Na2SO4), andconcentrated in vacuo. Evaporation of the solvent under reducedpressure gave the crude product. Purification by flash column chromatography(Silica gel 230-400 mesh, 1-10% ethyl acetate/ petroleumether) afforded the desired styrene as a colorless liquid. 5.1.2.1. Synthesis of 1-Methoxy-4-vinylbenzene (4a). Following theprotocol as cited earlier [27], compound 4a starting with 4-methoxybenzaldehyde (3c) (1 g, 7.34 mmol) was synthesized bygeneral procedure of Styrene preparation, with similar workup andpurified by flash chromatography on (230-400 mesh) silica. [Yield:91% (895 mg, colorless oil (Rf ≈ 0.7, PET/ EtOAc (9/1)].1H NMR: (500 MHz, CDCl3) δ (ppm) 3.82 (s, 3H), 5.13 (d,J = 11.2 Hz, 1H), 5.61 (d, J = 17.6 Hz, 1H), 6.67(dd, J1 = 10.8 Hz,J2 = 17.6 Hz, 1H), 6.87 (dd, J1 = 2 Hz, J2 = 9 Hz, 2H), 7.35 (d,J = 8.5 Hz, 2H).
References:
Adhikari, Susanta Sekhar;Banerjee, Rajkumar;Jinka, Sudhakar;Yousuf, Md [Bioorganic Chemistry,2020,vol. 98]
123-11-5
878 suppliers
$10.00/10g

637-69-4
244 suppliers
$8.00/1g
3319-15-1
116 suppliers
$10.00/1g

637-69-4
244 suppliers
$8.00/1g