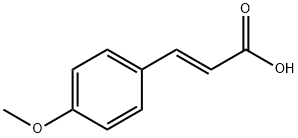
4-METHOXYCINNAMIC ACID synthesis
- Product Name:4-METHOXYCINNAMIC ACID
- CAS Number:943-89-5
- Molecular formula:C10H10O3
- Molecular Weight:178.18
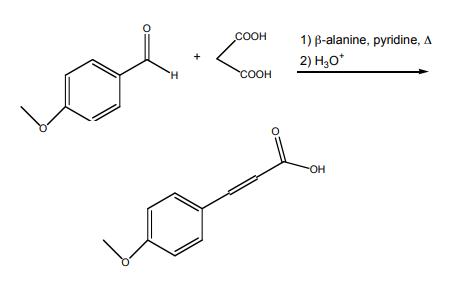
Synthesis of 4-methoxycinnamic acid
141-82-2
777 suppliers
$5.00/25g
123-11-5
878 suppliers
$10.00/10g

943-89-5
179 suppliers
$19.89/10g
Yield:943-89-5 98%
Reaction Conditions:
with piperidine in pyridine for 4 h;Reflux;
Steps:
Synthesis of (E)-3-(4-Methoxyphenyl)acrylic acid (7b)
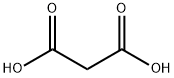
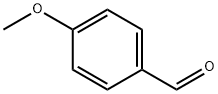
Malonic acid (1.24 g, 12 mmol) was added to a stirred solution of 6b (1.36 g, 10 mmol), pyridine(6 mL) and piperidine (0.6 mL). The reaction was stirred at reflux for 4 h. The mixture was then cooledto room temperature and poured into a solution of HCl (10 M in H2O, 80 mL) at 0 °C. The white solidthat formed was recovered by filtration and washed with water (200 mL) to afford the title compoundas white powder (1.33g, 98%). 1H NMR (300 MHz, CD3OD) δ 7.62 (d, J = 16.0 Hz, 1H), 7.58-7.47 (m,2H), 7.01-6.88 (m, 2H), 6.33 (d, J = 15.9 Hz, 1H), 3.82 (s, 3H). Data were in accordance with theliterature [28].
References:
Cornelio, Marinonio L.;Jones, Alan M.;Le Duff, Cécile S.;Povinelli, Ana Paula R.;Tang, Bridget;Zazeri, Gabriel [Molecules,2020,vol. 25,# 12]
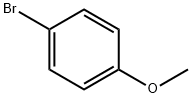
104-92-7
433 suppliers
$10.00/10g
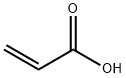
79-10-7
724 suppliers
$20.16/100 mL

943-89-5
179 suppliers
$19.89/10g

696-62-8
355 suppliers
$5.00/1g

79-10-7
724 suppliers
$20.16/100 mL

943-89-5
179 suppliers
$19.89/10g
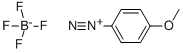
459-64-3
153 suppliers
$20.50/1g

79-10-7
724 suppliers
$20.16/100 mL

943-89-5
179 suppliers
$19.89/10g
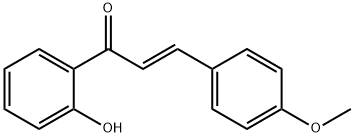
34000-29-8
2 suppliers
inquiry

943-89-5
179 suppliers
$19.89/10g