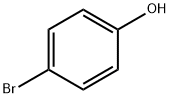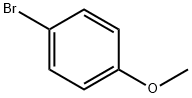
4-Bromoanisole synthesis
- Product Name:4-Bromoanisole
- CAS Number:104-92-7
- Molecular formula:C7H7BrO
- Molecular Weight:187.03
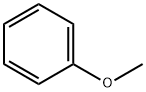
100-66-3
537 suppliers
$10.00/5G

104-92-7
433 suppliers
$10.00/10g
Yield:104-92-7 100%
Reaction Conditions:
with bis[1-methyl-3-(3-sulfopropyl)imidazolium] hexafluorotitanate;dihydrogen peroxide;sodium bromide in water at 25; for 3 h;Green chemistry;Reagent/catalyst;Concentration;Solvent;Temperature;
Steps:
2.3 General procedures for bromination of arenes using NaBr and H2O2 catalyzed by 1
General procedure: For the typical experiment, to 3 mL of water, anisole (or the other substrate, 5 mmol), NaBr (10 mmol), 1 (2.0 mmol), and 30% aqueous solution of H2O2 (10 mmol) were mixed sequentially in a single addition. The obtained mixture was stirred vigorously at room temperature. Upon completion of the reaction, the upper organic phase was separated by decantation and the left mixture was extracted by diethyl ether repeatedly (2 mL × 3 mL). The combined organic phase was analyzed by GC and GC-MS. The conversion of anisole was based on GC analysis with n-dodecane as the internal standard. The selectivity of the product was based on GC analysis using the normalization method. The GC yield was obtained on the basis of conversion × selectivity. The products were further identified by GC-MS. (0011) The left mixtures, containing the aqueous phase and the precipitated yellow solids, were added with the saturated Na2SO3 solution gradually to destroy the unreacted H2O2 until the KI-starch test paper changed to blue color. Afterwards, the obtained mixtures were recharged with anisole (5 mmol), NaBr (5 mmol), H2O2 (10 mmol), and H2SO4 (2.5 mmol) if required, for next run.
References:
Wang, Ling;Wang, Sa-Sa;Vo-Thanh, Giang;Liu, Ye [Journal of Molecular Catalysis A: Chemical,2013,vol. 371,p. 56 - 62]

104-94-9
503 suppliers
$9.00/1g

104-92-7
433 suppliers
$10.00/10g

696-62-8
353 suppliers
$5.00/1g

104-92-7
433 suppliers
$10.00/10g
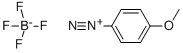
459-64-3
156 suppliers
$24.00/1g

104-92-7
433 suppliers
$10.00/10g