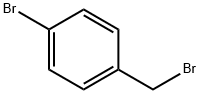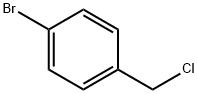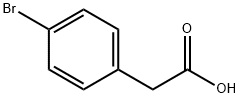
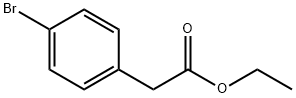
4-Bromophenylacetic acid synthesis
- Product Name:4-Bromophenylacetic acid
- CAS Number:1878-68-8
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
14062-25-0
248 suppliers
$6.00/5g

1878-68-8
505 suppliers
$6.00/10g
Yield:1878-68-8 93%
Reaction Conditions:
with water;sodium hydroxide in 1,4-dioxane; pH=10 - 14 at 60; for 2 h;
Steps:
19 Preparation of p-Bromophenylacetic Acid from p-Bromotoluene and Ethanol
p-Bromotoluene (2.72 g), ethanol (46 mg), di-tert-butyl peroxide (73 mg, 1 equivalent), and Pd(Xantphos)Cl2 (3.8 mg, 1 mol %) were added into a reaction kettle, into which 10 atm carbon monoxide was introduced. The reaction was heated to 120° C., and stirred at this constant temperature for 16 h. After the reaction was completed, carbon monoxide was discharged, and 89 mg ethyl p-bromoacetate was obtained by column chromatography, in a yield of 73%. 1HNMR (400 MHz, CDCl3) δ 1.23 (t, J=7.2 Hz, 3H), 3.56 (s, 2H), 4.12 (q, J=6.8 Hz, 2H), 7.15-7.17 (m, 2H), 7.43-7.45 (m, 2H); 13CNMR (100 MHz, CDCl3) δ 14.2, 40.8, 61.0, 121.1, 131.0, 131.6, 133.1, 171.1; HRMS (ESI) calcd. for C10H11BrNaO2 [M+Na]: 264.9835. found: 264.9837. The ethyl p-bromophenylacetate obtained was dissolved in 1,4-dioxane. 6 N sodium hydroxide solution was added, and the reaction was heated to 60° C. After 2 h of reaction, the pH value was adjusted to 1 by adding 2 N hydrochloric acid. After removing the organic solvent under reduced pressure, 73 mg product p-bromophenylacetic acid was obtained by extraction with ethyl acetate, and the yield of hydrolysis was 93%.
References:
Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences;Huang, Hanmin;Xia, Chungu;Xie, Pan US2013/303798, 2013, A1 Location in patent:Paragraph 0018; 0064; 0065

124-38-9
129 suppliers
$175.00/23402
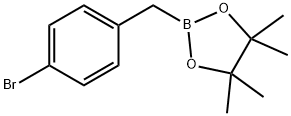
477841-90-0
31 suppliers
inquiry

1878-68-8
505 suppliers
$6.00/10g

124-38-9
129 suppliers
$175.00/23402
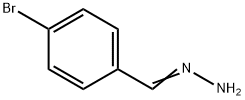
57477-93-7
2 suppliers
inquiry

1878-68-8
505 suppliers
$6.00/10g