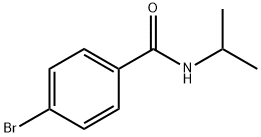
4-Bromo-N-isopropylbenzamide synthesis
- Product Name:4-Bromo-N-isopropylbenzamide
- CAS Number:336182-29-7
- Molecular formula:C10H12BrNO
- Molecular Weight:242.11
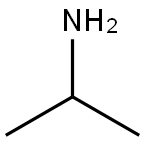
75-31-0
439 suppliers
$14.00/25mL
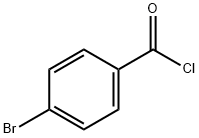
586-75-4
232 suppliers
$10.00/5g

336182-29-7
40 suppliers
$75.00/1g
Yield:336182-29-7 670 mg
Reaction Conditions:
with triethylamine in dichloromethane; for 0.166667 h;
Steps:
Preparation of 4-brom-M-isopropylfoen2amide
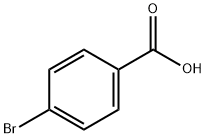
Preparation of 4-brom-M-isopropylfoen2amide 4-Bromobenzoic acid (630 mg, 3,00 mmol) was added to CHstCfz (10 mL). To this suspension was added oxalyl chloride (766 mg, 9.00 mmol) and 2 drops of DIVIF. The reaction was stirred until clear (approximately 1 hour). Then more oxaiy chloride (655 mg, 5.16 mmol) was added. No gas was liberated. To the reaction was added Et3N (3.4 mL, 24.0 mmol) followed by isopropylamine (1.02 mL, 12.0 mmol). The reaction was stirred for 10 minutes before it was quenched with 2 N HCI, extracted with DCM, dried and evaporated under reduced pressure. The crude product was purified by chromatography eiuting with petroium/EtOAc from 5/1 to give an off-white solid (670 mg, 92%). NMR (400 MHz, CDCI3): δ 7.64 (d, J - 8.8 Hz, 2H), 7.56 (d, J - 8.8 Hz, 2H), 5.92 (br s, 1 H), 4.32-4.27 (m, 1 H), 1.28 (d, J= 6.8 Hz, 6H).
References:
WO2015/74123,2015,A1 Location in patent:Page/Page column 50
586-76-5
489 suppliers
$10.00/1g

75-31-0
439 suppliers
$14.00/25mL

336182-29-7
40 suppliers
$75.00/1g
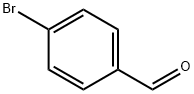
1122-91-4
703 suppliers
$9.00/10g

75-31-0
439 suppliers
$14.00/25mL

336182-29-7
40 suppliers
$75.00/1g
2700-30-3
139 suppliers
$5.00/1g
106-38-7
412 suppliers
$14.00/5g

336182-29-7
40 suppliers
$75.00/1g
99-73-0
240 suppliers
$10.00/1g
693-13-0
561 suppliers
$13.00/1ml

336182-29-7
40 suppliers
$75.00/1g